Clinical Applications of AS-OCT in Cornea & Refractive Surgery
AS-OCT has been widely used in the morphological evaluation of the anterior chamber. Anterior chamber depth (distance between corneal endothelium to crystalline lens pole), crystalline lens rise (CLR; distance between the anterior pole of the crystalline lens and the line joining two iridocorneal angles) and anterior chamber angle (with reference to the scleral spur) are the most commonly used parameters in AS-OCT.[15–17,21] These parameters can be used in presurgical planning of various anterior segment procedures, to analyze post-surgical chamber angle dynamics and in advanced intraocular lens (IOL) power calculations.[7,16] OCT has also been used to demonstrate ciliary body contraction, anterior chamber depth changes and lens movement during accommodation.[22,23]
AS-OCT in Phakic IOL Implantation
Phakic IOL is an emerging refractive surgery technique that has been used in the treatment of high refractive errors. Preoperative evaluation of anterior segment structures is very important in the evaluation of the phakic IOL candidate.[24] Recent updates in AS-OCT allow the surgeon to simulate the position of the phacic IOL before surgery. Postoperatively, AS-OCT is used in the examination of the position of the IOL and can be used to visualize the contact between the refractive implant and the crystalline lens.[21,25] The contact between the Implantable Collamer Lens and the crystalline lens is associated with development of anterior subcapsular opacification.[26]
Preoperative limits for iris-claw phacic IOL implantation are a minimum anterior chamber depth of 2.8 mm and a CLR of less than 300 µm. The risk of pigment dispersion is higher in cases with a CLR value higher than 600 µm. Another important criteria in phakic IOL is the clearance between the edge of the IOL and the endothelium, which must be at least 1.5 mm. In cases with IOL/endothelium distance less than 1 mm, explantation is required.[22] Because of these reasons, AS-OCT has been suggested as necessary for phakic IOL implantation as topography is for excimer laser surgery.[21]
Evaluation of the Cornea With AS-OCT After Cataract Surgery
AS-OCT imaging has been used to analyze the structure, integrity and configuration of the corneal incisions after cataract surgery.[27] These studies demonstrated that epithelial closure was completed at 1–8 days postoperatively.[28,29] Postoperative localized Descemet detachment was found in 40–82% of the patients on postoperative day 1.[30,31] Stromal hydration was associated with prolonged stromal swelling, which lasted for up to 7 days.[28–30] This result proves that the sealing effect of stromal hydration is much longer than clinically expected.[30] AS-OCT has revealed high frequencies of misalignment of the epithelial and endothelial side of the corneal incisions. As a result, early postoperative low intraocular pressure was suggested as a risk factor for wound leaking after phacoemulsification surgery.[31,32] Spectral domain AS-OCT has been used to acquire 3D architecture of the corneal wound and to make volumetric measurements.[33] Further advancements and studies are required with these prototype systems to better understand the architecture of the corneal incisions and their importance in the development of endophthalmitis.
Descemet membrane detachment (DMD) is a rare complication of anterior segment surgery.[34] DMD must be suspected in patients with persistent or unexpected postoperative corneal edema. Early diagnosis and surgical intervention with Descemetopexy has been suggested to improve the outcome in DMD. In cases with extensive edema, the diagnosis may be difficult with slit-lamp examination. In a case series with 14 patients, the diagnosis of DMD had to be confirmed with AS-OCT in 14% of the patients.[35]
Some of the retinal OCT systems can also be used in the evaluation of the anterior segment. Although the software of these systems is designed for the retinal nerve fiber layer and retinal thickness evaluation, they can be used to produce cross-sectional images of the anterior segment.[36] Stratus OCT™ (Carl Zeiss Meditec) has been used in corneal thickness measurements and morphological analysis in various conditions.[37–39] One of the latest software updates of Stratus OCT (version 6.0) includes corneal thickness measurements and the visualization of the anterior chamber angle. However, the resolution and distortion of the images limit the efficacy of Stratus OCT in the evaluation of the anterior segment (Figure 2).
Figure 2.
Slit lamp picture (A) and Stratus optical coherence tomography (B). Images of a patient with Descemet membrane detachment after phacoemulsification and intraocular lens implantation on postoperative day 3. White arrows demonstrate corneal edema (A) and Descemet membrane detachment (B).
AS-OCT in Descemet Stripping Automated Endothelial Keratoplasty
AS-OCT is a very useful tool in preoperative evaluation and management of patients undergoing Descemet stripping automated endothelial keratoplasty (DSAEK).[40] The most common postoperative complication in DSAEK is donor graft detachment. In these cases early recognition and management is mandatory to avoid graft failure. AS-OCT is used to confirm localized or total graft dislocation after DSAEK surgery (Figure 3). It is especially useful in cases with significant corneal clouding and limited anterior chamber visualization.[41] AS-OCT can also demonstrate the reason of graft failure in DSAEK. Residual host corneal Descemet membrane, eccentric trephination and inverse implantation of the donor can be demonstrated by the OCT images.[42–44] Serial monitoring of both donor and recipient corneas provides a reliable assessment of endothelial function and prognosis of the surgery.[40] AS-OCT also documented the cause of post-surgical hyperopic shift in DSAEK cases, which was induced by a high ratio of central graft thickness to peripheral graft thickness.[45] AS-OCT can also be used to demonstrate and to confirm the diagnosis of epithelial ingrowth after DSAEK.[46]
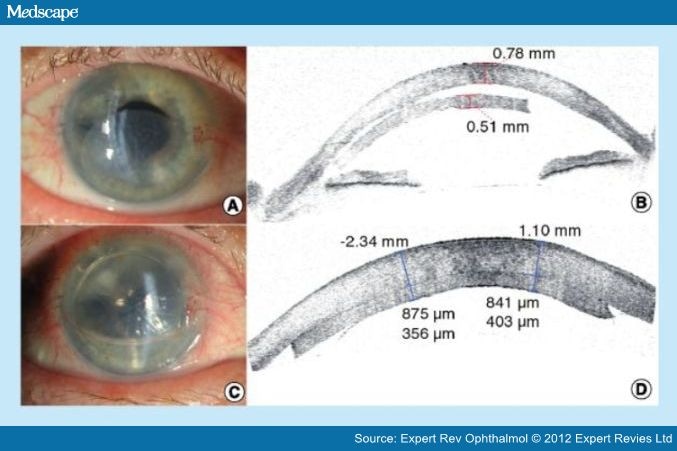
Figure 3.
Slit lamp pictures and Visante® optical coherence tomography images of a patient after Descemet stripping automated endothelial keratoplasty at postoperative week 1. (A) Slit lamp picture demonstrates detached Descemet stripping automated endothelial keratoplasty (DSAEK) graft. (B) Visante optical coherence tomography (OCT) image demonstrates detached DSAEK graft. Central corneal thickness is 780 µm and the thickness of the DSAEK graft is 510 µm. (C) Slit lamp picture 1 h after rebubbling. (D) Visante OCT image confirms reattachment of the DSAEK graft, 1 h after rebubbling. Central corneal thickness is measured as 875 and 841 µm. The thickness of the DSAEK graft is measured as 356 and 403 µm. Note the immediate decrease in the thickness of the donor stroma after rebubbling.
Expert Rev Ophthalmol. 2012;7(3):241-250. © 2012 Expert Reviews Ltd.