July 26, 2018 feature
New materials undergo solid-liquid phase transitions at room temperature
Researchers have developed the first materials that can permanently change from solid to liquid, or vice versa, when exposed to light at room temperature, and remain in the new phase even after the light is removed. The researchers also demonstrated that the light can be used to draw liquid designs in a solid material or solid designs in a liquid material, creating stable materials that are part solid and part liquid. The new materials have potential applications for 3-D printing, molding, and on-demand recycling, among other uses.
The researchers, led by Brady Worrell, Christopher Bowman, and coauthors at the University of Colorado, Boulder, have published a paper on the materials with photoswitchable phases in a recent issue of Nature Communications.
As we see in everyday life, conventional materials switch phases due to changes in temperature or pressure. For example, solid ice can be turned into liquid water by heating or—less commonly—by increasing the pressure (a higher pressure lowers the melting point, causing the ice to melt at colder temperatures than normal).
Certain polymers, however, are permanently solid—even when exposed to extreme changes in temperature or pressure, they never become liquid. These materials, which are called covalently cross-linked polymers, can be modified so that an external stimulus such as light or heat causes them to switch from solid to liquid. However, this is only a temporary change, in which the polymer reverts back to its solid form as soon as the stimulus is removed.
In the new study, the researchers presented two new polymers, one which starts as a solid and can be converted into liquid, and the other which starts as a liquid and can be converted into a solid. The polymers are the first materials of any kind that can undergo a permanent phase change in response to a stimulus other than temperature or pressure (in this case, light).
The solid and liquid polymers both switch phase when irradiated by UV light with a 365-nm wavelength for about five minutes. However, the light affects the two materials differently. The liquid polymer initially contains a base that promotes a stress-relaxing thiol-thioester exchange reaction, which causes the polymer to act like a fluid, but the solid does not initially contain this base. When the solid polymer is exposed to light, the light releases a catalyst that releases the base, promoting the stress-relaxing reaction and converting the solid to a fluid. On the other hand, when the liquid polymer is exposed to light, the light releases a different catalyst that releases acid, neutralizing the base and halting the stress-relaxing reaction, which converts the liquid polymer into a solid.
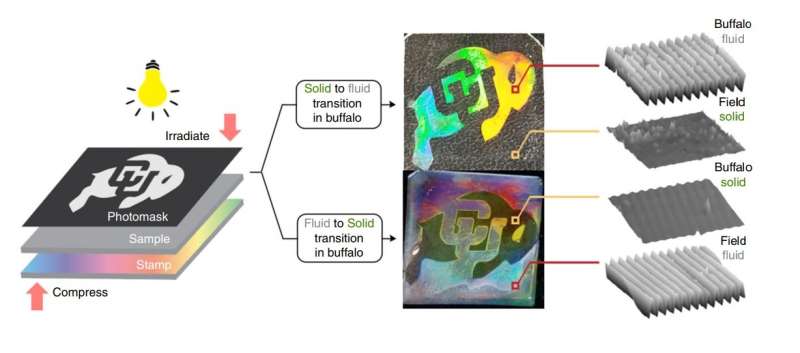
Using light instead of temperature or pressure to control the phase changes makes it possible to exert exquisite spatial control over these phase changes, allowing the researchers to define separate solid and liquid regions in a single material. To demonstrate, the researchers used nanoimprint lithography to design a photomask in the shape of a buffalo (the University of Colorado Boulder mascot). By using the two different wavelengths of light, they could make either a liquid buffalo on a solid background or a solid buffalo on a liquid background. Despite consisting of both liquid and solid, the material is stable and the liquid and solid portions remain permanently separate.
The researchers expect that, in the future, these abilities will open the doors to a variety of new applications where polymers are used.
"In a broad context, the thiol-thioester exchange in network polymers allows for wide-ranging application in a variety of fields," Worrell told Phys.org. "This material effectively bridges the gap between thermoplastics and thermosets at very low operating temperatures, allowing for recycling, repurposing or remolding (thermoplastic behavior) and on-demand application to a substrate (thermoset behavior). This material will therefore likely have appeal in smart coatings applied on-demand where environmental stresses limit effectiveness."
More information: Brady T. Worrell et al. "Bistable and photoswitchable states of matter." Nature Communications. DOI: 10.1038/s41467-018-05300-7
Journal information: Nature Communications
© 2018 Phys.org