Case Report
A 48-year-old electrophysiology technician initially presented with a history of metal allergy to costume jewelry, earrings, watches, hooks, snaps, and the metal nose clasp on her work mask. She had a personal and family history of psoriasis as well as a personal history of dorsal hand and foot eczema. Needing a cap for one of her front teeth, she was referred by her dentist for patch testing.
Patch testing was performed with a standardized technique with Finn Chambers (Epitest Ltd Oy, Tuusula, Finland) on Scanpor tape (Norgesplaster Alpharma A/S, Vennesla, Norway). The patches remained in place for 48 hours, and test sites were evaluated at 48 hours and again at 96 hours after initial placement, according to North American Contact Dermatitis Group (NACDG) guidelines.[1]
A positive reaction was interpreted in the manner previously reported by the NACDG. In summary, a grade of 1, 2, or 3 was used to document the intensity of positive patch-test reactions. Positive reactions consist of erythematous papules, vesicles, a spreading eruption sometimes with crusting and ulceration, or all three of these conditions. The clinical relevance of a positive patch-test reaction was determined by combining the patient's history and physical examination findings.[1,2]
The patient was patch-tested with the standard series of 65 allergens (Chemotechnique Diagnostics, Malmö, Sweden). She was also tested with supplemental series, including Chemotechnique's metal series, and with povidon-iodine, tincture of benzoin, and some of her own products and devices, such as the mask and gloves she wore at work.[2] The results at 96 hours showed positive reactions to cobalt chloride (+++) and nickel sulfate (+++).
A year later, the patient needed total knee arthroplasty. Arthroscopy showed stage III osteoarthritis of the right knee and stage IV osteoarthritis with significant deformity and instability in the left knee. The orthopedic surgeon was aware of the patient's contact allergy to nickel and cobalt chloride and referred the patient back to the patch test clinic for an opinion on which metal would be least likely to cause a problem for her knee replacement. Stainless steel, commonly used in orthopedic implants, contains nickel, to which the patient was allergic. The alloy consists of 15 to 20% chromium, 0% cobalt, and 8.5 to 14% nickel, but nickel content can be as high as 35%.[3,4] Cobalt-chrome-molybdenum alloys such as Vitallium are also favored in orthopedic surgeries. Vitallium contains mostly cobalt and is approximately 30% chromium and 5% molybdenum.[5] It was suggested that a titanium prosthesis would be the safest option, given the low incidence of reactions to this metal in the general population.[4,6] Unfortunately, titanium femoral components in total knee replacements have been prone to premature and severe failure and were no longer favored for joint replacements. In addition, the orthopedic surgeon preferred using a stainless steel or cobalt-chrome prosthesis because the patient's heavy weight and young age would require a robust joint. The patient was patch-tested again with the NACDG standard series of 65 allergens, metals in the dentistry series, and the metal series. The results at 96 hours were as follows: cobalt chloride, +++; nickel sulfate, +++; palladium, ++; thiourea mix, +++; thimerosal, +++; and quaternium-15, ++ (Figure 1, Figure 2 and Figure 3).
Figure 1.
Patch-test reactions to nickel sulfate and cobalt chloride.
Figure 2.
Patch-test reaction to nickel sulfate.
Figure 3.
Patch-test reactions to nickel sulfate and cobalt chloride.
Because the patient needed the knee replacement and because she had documented cobalt and nickel allergy, the possibility of doing subcutaneous test implants was considered. Opinions were sought from the NACDG and from other dermatologists who had expertise in metal allergy as to the prognostic value of subcutaneous implantation of cobalt-chrome and titanium samples to determine if the patient would react to her prosthesis. Their responses were mixed. There were no guidelines for doing an implant test (for example, how deep to place the implant and how long to leave it in the body).
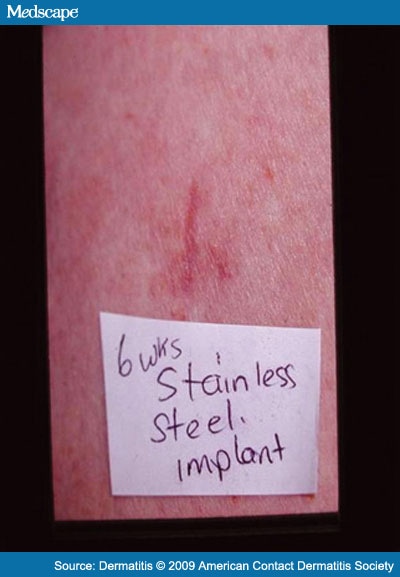
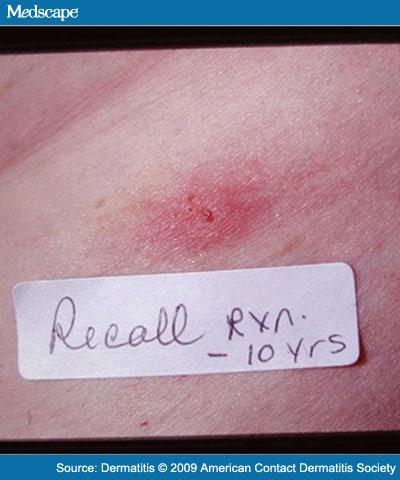
We decided to test with subcutaneous implants. In February 2000, two 1-inch round test pieces of two different metals were inserted subcutaneously. A cobalt-chrome piece was inserted into the right anterior thigh, and a titanium piece was inserted into the left anterior thigh. There were no local reactions at the sites of implantation (Figure 4 and Figure 5). At week 6, the patient developed a small annular eczematous patch on her left midback and a mild linear eczematous patch on her left lower leg. The eczematous patch on the left lower leg was at the site of an old fracture that had previously had a metal (likely stainless steel or Vitallium) plate and screw for a number of years (now removed) and was presumed to be a recall reaction (Figure 6 and Figure 7).
Figure 4.
Lack of reaction at the site of the cobalt-chrome subcutaneous implant (incorrectly labeled stainless steel).
Figure 5.
Lack of reaction at the site of the titanium subcutaneous implant.
Figure 6.
Recall reaction at the old site of previous orthopedic hardware (presumed stainless steel or Vitallium) removed 25 years ago.
Figure 7.
Recall reaction at the old site of previous orthopedic hardware (presumed stainless steel or Vitallium) removed 10 years ago.
In October 2000, left knee total arthroplasty surgery was performed with a Sigma knee prosthesis (Depuy/Johnson and Johnson, DePuy Orthopedics, Inc. Warsaw, IN) composed of a femoral component made of cobalt chrome (essentially identical to Vitallium), a titanium tibial tray component, and a polyethylene spacer. Table 1 shows the composition of the Sigma knee prosthesis.
The patient experienced intermittent erythema, swelling, and heat at the surgery site on the left knee for 4 weeks postoperatively. There was no fever or systemic toxicity. The erythema was treated as presumed infection but did not respond to antibiotics. One month after surgery, the patient developed an eczematous patch on the left knee over an old suture line and near the site of the prosthesis. A small eczematous patch also developed at the old fracture site on the left lower leg where the metal plate had been removed. The eczematous eruption was treated with halobetasol propionate ointment daily for 2 months, which resulted in some improvement but not clearance. The patient, however, was very happy and comfortable overall; she experienced significantly decreased pain in her left knee and marked improvement in ambulation.
In October 2001, a cobalt-chrome prosthesis was used to replace the right knee. Two months postoperatively, she developed an acute contact dermatitis on the anterior right knee. The reaction spread onto her thighs and abdomen, and there was a recall acute contact dermatitis at the old fracture site on the left lower leg. This reaction was treated with halobetasol ointment and triamcinolone ointment, with moderate success.
Over the next 8 years, the patient experienced episodic flares of acute contact dermatitis on both knees and the left ankle. These flares have continued into the present, occurring every 1 to 2 months and lasting approximately 2 weeks. The patient was treated with triamcinolone ointment and diphenhydramine and was put on a nickel-free diet, but she continued to have ongoing reactions (Figure 8). Overall, however, the patient's quality of life had improved significantly with her increased mobility, and she was comfortable managing her skin flares symptomatically.
Figure 8.
Ongoing episodic flares of contact dermatitis from bilateral cobalt-chrome knee replacements.
Dermatitis. 2009;20(2):E4-E9. © 2009 American Contact Dermatitis Society
Cite this: An Interesting Case of Joint Prosthesis Allergy - Medscape - Mar 01, 2009.


























Comments