The US Food and Drug Administration (FDA) has approved the first rapid handheld blood test for suspected mild traumatic brain injury (TBI), the company has announced. The test yields results within 15 minutes.
Manufactured by Abbott Laboratories, the i-STAT Alinity TBI plasma test measures glial fibrillary acidic protein (GFAP) and ubiquitin carboxyl–terminal hydrolase L1 (UCH-L1) in plasma.
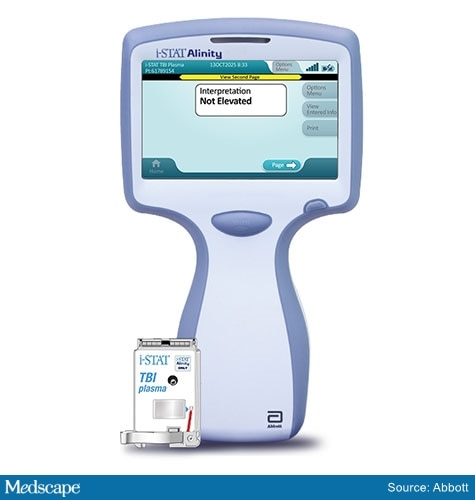
The i-STAT Alinity TBI plasma test .
Elevated concentrations of these two complimentary biomarkers are tightly correlated to brain injury, with 95.8% sensitivity and >99% negative predictive value, the company said in a news release.
The test was developed in collaboration with the US Department of Defense.
Each year in the United States, nearly five million people are evaluated in the emergency department for suspected TBI. Such a blood test could eliminate wait time in emergency departments and could reduce unnecessary CT scans by up to 40%, the company says.
"Healthcare providers have been waiting for a blood test for the brain, and now we have one," Beth McQuiston, MD, medical director for Abbott's diagnostics business, said in the news release.
"You can't treat what you don't know, and now physicians will be equipped with critical, objective information that will help them provide the best care possible, allowing patients to take steps to recover, prevent reinjury, and get back to doing the things they care about most," said McQuiston.
Abbott's TBI test requires a small blood sample drawn from the arm. Plasma is extracted from the sample with a centrifuge and is applied to the test's cartridge, which is then inserted into the handheld device.
Abbott is also working on a rapid whole blood test, which would eliminate the need for the separation of plasma. The test could be used outside of healthcare settings where people suffer head injuries and need a quick evaluation, such as sporting events.
"Evaluating brain injuries is complex ― and research shows that we only catch about half of those who show up to the hospital with a suspected TBI," Geoffrey Manley, MD, PhD, vice chair of neurologic surgery at the University of California, San Francisco, said in the news release.
"And beyond those who go to the hospital for a suspected TBI, many more never do. A test like this could encourage more people to get tested after a head trauma, which is important, because not receiving a diagnosis can be dangerous and may prevent people from taking the necessary steps to recover safely," said Manley.
For more Medscape Neurology news, join us on Facebook and Twitter.
Cite this: FDA Clears First Rapid Handheld Blood Test for Concussion - Medscape - Jan 14, 2021.











Comments