Abstract and Introduction
Abstract
The understanding of the interactions between biological systems and nanoengineered devices is crucial in several research fields, including tissue engineering, biomechanics, synthetic biology and biomedical devices. This review discusses the current knowledge of the interactions between bacteria and abiotic nanostructured substrates. First, the effects of randomly organized nanoscale topography on bacterial adhesion and persistence are described. Second, the interactions between microorganisms and highly organized/ordered micro- and nano-patterns are discussed. Finally, we survey the most promising approaches for the fabrication of silver polymeric nanocomposites, which have important applications as antimicrobial materials. The advantages, drawbacks and limitations of such nanotechnologies are critically discussed in view of potential future applications.
Introduction
Bacterial surface contamination – the adhesion, persistence and colonization of surfaces by bacteria – is increasingly recognized as detrimental to health and society.[1–4] Besides impacting food safety, biofilm growth (arising from surface contamination) distresses economic sectors, such as sea transport (biofilms promote the binding of aquatic invertebrates onto the immersed sections of ships), manufacturing (biofilms stimulate corrosion processes) and health/medicine, leading to immense human and economic costs.[1–9] It is impossible to provide a universal description of bacterial biofilms. From a chemical point of view, biofilms consist of a complex mixture of exopolysaccharides, DNA and catalytic proteins, which are secreted by microorganisms after their adhesion onto surfaces, and whose biological functions are largely unknown.[8,10–12] Biologically, it represents an external digestive system that allows extracellular enzymes (secreted by bacteria) to localize in close proximity to cells, enabling the metabolization of the solid biopolymers present in the nearby environment.[8] From a physical point of view, a biofilm is form of slime matrix with specific robustness, viscosity and strength that varies depending on the particular strain of microorganism(s) and the specific environmental conditions.[13–15] Ecologically, biofilms represent the preferred niche where almost all bacteria live;[16,17] the evolutionary mechanisms led them to grow as a sessile adherent community, rather than as a dispersed, planktonic form. There is evidence of bacterial biofilm formation even in 3-billion-year-old African fossils,[18] suggesting that such living forms promoted survival in the harsh environmental conditions of Earth at that time. From a genetic viewpoint, sessile bacteria have very different profiles of gene and protein expression, compared with their planktonic counterparts.[19–22] Hence, biofilms could represent a complex population of unicellular microorganisms that live, behave and interact with each other and the environment like a quasi-multicellular organism.
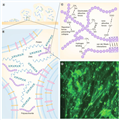
Figure 1 illustrates the most important features of biofilm formation, development and composition.[3,8] The most characterizing aspect of biofilms is, therefore, their 'heterogeneity'.[19] Another characteristic is the high durability in terms of resistance to mechanochemical or physical stress stimuli (i.e., UV radiation, antibiotic treatment and scraping). As such, it is widely accepted that the most effective way to prevent biofilm formation is a priori suppression of bacterial adhesion, rather than a posteriori chemical- or drug-based treatment. Great effort has been put into designing substrates that prevent the early stage of bacterial adhesion.
Figure 1.
The complex extracellular polymeric substance produced by adherent bacteria.
(A) Dynamic evolution, over time, of a bacterial biofilm onto abiotic substrates. After the adhesion of bacteria, the microcolonies start to divide and grow, and the biofilm matrix is secreted. During biofilm expansion, other environmental microorganisms can be involved and may became part of the colony, thus strengthening the overall community. At the same time, sessile bacteria can be dispersed throughout the surrounding surface. (B) Principal components of the biofilm matrix: polysaccharides, proteins and DNA are distributed between the cells in a heterogeneous (and almost unique) pattern. (C) Classes of physicochemical interactions that regulate the stability of the extracellular polymeric substance matrix.115 (D) SYTO9 staining of a biofilm produced by a 4-day-old culture of the γ-proteobacterium strain F8.
Reproduced with permission from [8].
The main factors influencing bacterial adhesion are the physicochemical properties of the substrate material and microorganisms, and the environmental conditions.[23–25] Many investigations have focused on chemically modifying the abiotic surface to repel bacteria.[26–30] However, chemical modification has several drawbacks, such as reduced efficiency once the compounds/drugs are released, potential toxicity and the possible occurrence of local immunogenicity in vivo. It should also be considered that several anti-infective materials for medical use have been functionalized with antibiotics. This could contribute to the current spreading of antibiotic resistance, which is creating great concerns worldwide and making treatment of infection difficult.[31–34] Thus, recent attention has shifted toward exploiting surface topography. In this regard, nanotechnology offers new methods and techniques to design innovative surfaces with specific and highly controlled surface nanotextures.
The aim of this article is to provide a critical overview of the antibacterial effects of nanoderived substrates. The first section describes the effect of random nanoroughness on bacterial adhesion and persistence; the second section focuses on highly ordered micro- and nano-patterns; and the third section discusses silver polymeric nanocomposites (both synthetic and natural/bioinspired), a particularly promising class of antibacterial materials. For each topic, we critically address the limitations, especially from the biological standpoint, as well as discussing open issues and the future outlook.
Nanomedicine. 2013;8(5):807-821. © 2013 Future Medicine Ltd.