Abstract and Introduction
Introduction
The National Electronic Disease Surveillance System (NEDSS) is a web-based system that uses standard health information technology (IT) codes to integrate disease surveillance systems, enabling them to transfer public health, laboratory, and clinical data securely from health-care providers to public health departments.[1] Each jurisdictions' system consists of a base system and modules that can be used for specific surveillance purposes. States also use NEDSS-like or other electronic systems to conduct surveillance on specific diseases or conditions.* Until recently, no assessment had been done to describe the status and characteristics of state electronic disease surveillance systems. The Council of State and Territorial Epidemiologists (CSTE) conducted such an assessment in August 2007 in all 50 states. This report presents the results of that assessment, which indicated that, in 2007, state electronic disease surveillance systems varied widely and were in various stages of implementation. Each state had either custom-built systems or purchased systems that were customizable, with associated disease modules to meet its own surveillance needs. As interoperability becomes the standard for electronic data sharing, more states will face customization costs and the need to hire more technical specialists who can manage health information and exchange. Further collaboration and support from surveillance and health-care IT stakeholders with public health will be needed to improve the efficacy and quality of electronic disease surveillance systems.
States have developed their electronic disease surveillance systems in a multitude of ways, and states use a combination of vendor products, CDC electronic systems, and state-developed surveillance systems. Some electronic systems are disease specific (e.g., human immunodeficiency virus [HIV]/acquired immunodeficiency syndrome [AIDS] and tuberculosis [TB]), and others serve a particular purpose (e.g., outbreak management, electronic laboratory reporting).† In 2000, CDC developed the NEDSS Base System, a platform for disease-specific modules, which it supports and provides to states for use in surveillance. Except for the hardware costs, states using the NEDSS Base System generally incur only commercial software maintenance fees and licenses. States and vendors have developed enhancements that facilitate surveillance through electronic laboratory reporting, geographic information mapping, and outbreak management software.
In 2007, the NEDSS and Architecture Subcommittee of CSTE developed a survey to assess the status, progress, and features of the various electronic surveillance systems used by states nationwide. CSTE distributed the questionnaire electronically to NEDSS project managers or their designees in each state, who completed a series of multiple-choice questions on the operational status and integration levels of their systems and provided additional data on how their system software was developed. The questionnaire also asked respondents to provide vendor information and to comment on other aspects of their systems.
The assessment collected data on five NEDSS Base System, NEDSS-like, or separate, web-based electronic surveillance systems used by most states: communicable human diseases, HIV/AIDS, lead exposure, sexually transmitted diseases other than HIV/AIDS, and TB. The questionnaire also collected information about IT enhancements, such as electronic laboratory reporting, geographic information mapping, Master Patient Index,§ and outbreak management systems¶ to assess their level of potential integration with other systems and their development status.
For the assessment, CSTE defined "interoperability" as the extent to which the configuration of a surveillance system allowed exchange of information by electronically connecting various stand-alone, disease-specific modules within the state or allowed exchange of information among dissimilar systems in different states. CSTE defined "integration" as the extent to which a system included all of the separate disease modules in the same system.
All 50 states responded to the assessment questionnaire, but not all states answered all questions. Sixteen (32%) states reported using the NEDSS Base System as their general communicable disease electronic surveillance system. The remaining 34 (68%) states reported using some combination of commercial, CDC, or state-developed electronic surveillance systems to meet their needs. Among the 50 states, 39 (78%) reported that at least one aspect of their surveillance systems was under development or planned, and 35 (70%) reported that their system could send a message about communicable disease in Health Level Seven (HL7)** format to CDC. Among the 40 states with an operational electronic surveillance system (i.e., fully functional and currently in use) for general communicable disease surveillance, 23 (58%) reported having an integrated system, 15 (38%) had stand-alone systems, and two (5%) did not designate whether their system was integrated or stand alone. The 10 states without fully functional and operational systems were in the process of developing one or more aspects of their electronic disease surveillance system at the time of the assessment.
Results of the assessment indicated that web-based HIV/AIDS surveillance systems were mostly stand-alone systems (Table 1). Among 41 states, 17 (41%) reported having an operational and fully implemented web-based lead poisoning surveillance system. Among the 22 states with fully functional, web-based TB case-reporting systems, 11 (50%) were integrated and 11 (50%) were stand-alone systems. Eighteen (36%) of 50 states had developed their TB surveillance modules (TB case-management, TB case-reporting, and latent infection tracking) in-house, and TB surveillance systems in seven (14%) states were vendor developed. Fourteen (28%) of 50 states used a CDC-developed solution to meet their TB surveillance needs.
The three most commonly integrated modules were the automated electronic laboratory reporting module, the web-based manual electronic laboratory reporting module, and the Master Patient Index module. Automated and web-based manual electronic laboratory reporting modules differ in the labor involved in entering the information into the system. Automated systems do not require data entry into an online system, whereas the web-based electronic laboratory reporting modules do. These more recently developed modules were more commonly integrated into the general communicable disease systems than were stand-alone HIV/AIDS and TB surveillance modules. Among the 50 states, eight reported having functional outbreak management systems, among which four each had stand-alone systems and integrated systems. Outbreak management systems in 20 states were either under development or targeted for future development, and 22 states did not report having an outbreak management system. Four states reported having source code of the general communicable disease surveillance system available to the general public for use or modification from its original design free of charge and were willing to share state written code with any interested state or local health departments.
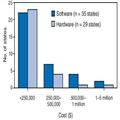
Among the 50 states, 13 (26%) reported achieving interoperability among two or more surveillance modules, and seven (14%) reported future plans for interoperability. Twenty-eight (56%) states were acquiring new technology and software and hardware required by the system to support interoperability, and one state did not respond to the question. Combined software and hardware costs ranged from $250,000 to $1 million for electronic disease surveillance systems, without additional customization. For most states, software costs were <$250,000 (Figure). The 29 states reporting hardware costs indicated approximate costs of <$250,000 to enable interoperation with another state system, without customization. Additional costs cited by respondents included annual licensing fees from software developers/vendors, security customization fees, and costs associated with tailoring a surveillance system to state or local needs (ranging from $20,000 to $50,000). The assessment indicated no clear association between software cost and state population.
Figure.
Approximate costs to deploy software and hardware for state electronic disease surveillance systems — United States, 2007
States averaged two to three (range: 1–12) full-time equivalents (FTEs) for each IT role (Table 2). States with mid-sized to large populations reported more FTEs in each IT role than did smaller states, but most states generally had no more than four FTEs for each IT role. These roles were not discrete, and FTEs might have performed overlapping duties among the various roles.
Morbidity and Mortality Weekly Report. 2009;58(29):804-807. © 2009
Cite this: Status of State Electronic Disease Surveillance Systems — United States, 2007 - Medscape - Jul 31, 2009.







Comments