Approach Considerations
The treatment of glioblastomas remains difficult in that no contemporary treatments are curative. [187] While overall mortality rates remain high, improved understanding of the molecular mechanisms and genetic mutations combined with clinical trials are leading to more promising and tailored therapeutic approaches. Multiple challenges remain, including tumor heterogeneity; tumor location in a region where it is beyond the reach of local control; and rapid, aggressive tumor relapse. Therefore, the treatment of patients with malignant gliomas remains palliative and encompasses surgery, radiotherapy, and chemotherapy. Because none of these treatments is curative, the National Comprehensive Cancer Network (NCCN) recommends clinical trials for eligible patients. [22]
Treatment should be tailored to each patient based on age, functional status, and goals of care. [58] Palliative care should be integrated early in the clinical course, and supportive care may be the best option for some patients (eg, those with large or multifocal lesions who have a low Karnofsky Performance Scale score). [23] See Brain Cancer Treatment Protocols for summarized information.
Upon initial diagnosis of glioblastoma (GBM), standard treatment consists of maximal surgical resection, radiotherapy, and concomitant and adjuvant chemotherapy with temozolomide. [58, 188, 22] At some institutions, transferring the patient to another facility may be necessary if the proper consultations cannot be obtained. In most cases, surgical resection can be performed on an urgent, but not emergent, basis. Patients with glioblastomas who undergo surgical resection typically spend the night after surgery in an intensive care unit, followed by an inpatient stay. The final length of stay depends on each patient's neurologic condition.
Postoperative antibiotics usually are continued for 24 hours, and deep vein thrombosis prophylaxis is continued until patients are ambulatory. Anticonvulsants are maintained at therapeutic levels throughout the inpatient stay, while steroids are reduced gradually, tailored to each patient's clinical status. Many patients benefit from occupational therapy and physical therapy or rehabilitation.
While in the hospital, patients should receive postoperative imaging to verify the extent of surgical resection, preferably within 24-48 hours of surgery, using magnetic resonance imaging (MRI) with and without contrast. [58] Contrast enhancement during this period accurately reflects residual tumor. MRI should include diffusion-weighted sequences for detection of perioperative ischemia. [189] If not performed preoperatively, complete evaluations by consulting physicians, including a neuro-oncologist and radiation oncologist, should be considered postoperatively.
For patients older than 70 years, less aggressive therapy with radiation or temozolomide (TMZ) alone is sometimes employed, as these patients tend to be less tolerant of toxicities. [190, 42, 191, 192] A study by Scott et al found that elderly patients with glioblastoma who underwent radiotherapy had improved cancer-specific survival and overall survival compared with those who did not undergo radiotherapy treatment. [193]
Evidence suggests that in patients over 60 years old, treatment with TMZ is associated with longer survival than treatment with standard radiotherapy, and for those over 70 years old, TMZ or hypofractionated radiotherapy is associated with longer survival than treatment with standard fractionated radiotherapy; the improvement in survival with TMZ is enhanced in patients with MGMT promoter methylation. [43] Data from a randomized phase III trial suggest that lomustine-temozolomide plus radiotherapy might be superior to TMZ chemoradiotherapy in newly diagnosed glioblastoma with methylation of the MGMT promoter. [194]
In their highly influential randomized phase III clincal trial comparing combined therapy with TMZ and radiation versus radiation alone in patients with glioblastoma, Stupp et al found that TMZ plus radiotherapy was associated with a greater median and 2-year survival, [31] and survival in the combined therapy group continued to exceed that of radiation alone across all clinical prognostic subgroups throughout the 5-year follow up. [45]
Glioblastomas virtually always recur after standard therapy, with a median time to recurrence of 6.7 months following initial treatment. [152] There is no established standard-of-care salvage therapy, and the NCCN guidelines recommend clinical trials. [22] While surgery may be appropriate in patients with symptomatic or large lesions, only gross total resection (GTR) is associated with a survival benefit. [26, 27, 29] Various other options have been employed, including temozolomide rechallenge, nitrosoureas, bevacizumab, re-irradiation, tumor treating fields, and various investigational therapies. [44, 195, 196] Unfortunately, none of those has been shown to prolong survival in randomized controlled trials.
Surgical Care
Surgical care in glioblastoma should be individually tailored, taking into consideration the indications, risk-benefit ratio, and prognostic impact for each patient. Because glioblastoma cannot be cured with surgery, the surgical goals are to establish a pathologic diagnosis, relieve mass effect, and, ideally, achieve a gross total resection (GTR) to facilitate adjuvant therapy while preventing new permanent neurologic deficits that might jeopardize independence, decrease quality of life, and increase the risk of additional complications that could delay or even preclude additional therapy. [23] While neurologic deficits can sometimes be predicted preoperatively, patients should be counseled that neurosurgical procedures are always associated with some unpredictable risks. [23]
Biopsy
If the patient should refuse surgery or if surgery is not feasible due to the patient’s medical comorbidities and/or involvement of eloquent cortex, a stereotactic or open biopsy should still be performed for histologic diagnosis and molecular testing, which can guide subsequent therapy. [23, 197, 59] To obtain sufficient material for accurate diagnosis and grading, the surgeon should biopsy contrast-enhancing regions of solid tumor mass that contain viable tumor cells, ideally avoiding necrotic areas and surrounding healthy neural tissue. [58]
Extent of Resection
Historically, tumor extent was largely defined using T1-weighted MRI with contrast. [20] However, because glioblastoma is highly invasive, with infiltrating cells extending beyond the main, contrast-enhancing tumor mass, non–contrast-enhancing tumor should also be included in the target resection volume. [58, 162] The goal for glioblastoma surgery is the maximal resection that is safely feasible and leaves the smallest amount of residual postoperative enhancing tumor. [198]
While no randomized clinical trials have been conducted to determine the optimal extent of surgery in glioblastoma, gross total resection (GTR) is generally recommended, as several retrospective studies have shown that GTR is associated with improved progression-free and overall survival in both newly diagnosed [25, 26, 27] and recurrent glioblastomas [28, 29] in both young and elderly patients [27, 30] regardless of molecular status. [24] A sampling of these studies is discussed below:
-
In an early study by Ammirati et al, patients with high-grade gliomas who underwent gross total resection had a 2-year survival rate of 19%, while those who underwent subtotal resection had a 2-year survival rate of 0%. [199]
-
In a study of 92 patients by Keles et al, total tumor resection without any residual disease resulted in a median survival of 93 weeks, whereas the greatest volume of residual tumor (> 20 cm 3) and smallest percent of resection (< 25%) gradually shortened survival to 50 weeks and 31 weeks, respectively. [145]
-
In a study of 416 patients by Lacroix et al, gross total resection—defined as > 98% on MRI—conferred a mean survival advantage of approximately 4-months over subtotal resection (13 vs. 8.8 mo). [143]
-
Similarly, a meta-analysis of 28 studies found that gross total resection conferred a mean survival advantage of 3 months over subtotal resection (14 vs. 11 mo). [144]
-
Li and colleagues compared the survival of patients who received 100% resection of contrast-enhancing tumor (with or without additional resection of the surrounding FLAIR abnormality region) with that of patients who received 78% to < 100% resection of enhancing tumor. The median survival time for patients who underwent complete resection (15.2 months) was significantly longer than that for patients who underwent less-than-complete resection (9.8 months; P < 0.001). Furthermore, patients who underwent resection of ≥ 53.21% of the surrounding FLAIR abnormality beyond the 100% resection of enhancing tumor achieved significant prolongation of survival (median survival time 20.7 mo). [200]
-
In a study of 761 patients, Molinaro et al found significantly greater overall survival among patients younger than 65 years who received 100% resection of contrast-enhancing tumor and 90% resection of non-contrast-enhancing tumor, resulting in no more than 5.4 mL of residual non–contrast-enhancing tumor. Overall survival in this group was 37.3 months, versus 16.5 months in comparably young patients who had more than 5.4 mL of residual non–contrast-enhancing tumor after resection. [24]
Surgical Adjuncts
Numerous preoperative and intraoperative surgical adjuncts have been developed to maximize the extent of resection while minimizing the risk of new neurologic deficits. These include preoperative imaging studies, such as functional MRI (fMRI) and diffusion tensor imaging (DTI) fiber tracking, which are particularly useful when resecting tumors near or within eloquent brain regions. [21, 201, 202] Similarly, awake craniotomy with evoked potentials, electromyography, or brain mapping can be used to monitor and preserve language and other essential cognitive functions when eloquent cortex is involved. [203] Intraoperative MRI may help identify residual tumor and optimize the extent of resection, [204] and intraoperative ultrasound appears to be associated with preservation of quality of life via prevention of new neurologic deficits. [205]
In 2017, the fluorescent dye 5-aminolevulinic acid (5-ALA; Gleolan) was approved by the US Food and Drug Administration (FDA) as an adjunct for visualizing malignant tissue during surgery. [206] Since then, fluorescence-guided surgery (FGS) has been increasingly used to facilitate more complete and precise resection. A randomized trial comparing FGS with 5-ALA to conventional microsurgery with white light found that patients who received FGS had a 6-month progression-free survival (PFS) rate of 46.0%, whereas patients who received conventional surgery had a 6-month PFS rate of 28.3%. [207] Despite those promising data, widespread utilization of FGS has been limited by the cost of 5-ALA and the need for specialized equipment. An alternative fluorescent contrast agent, tozuleristide (BLZ-100), is currently undergoing clinical trials for use in FGS. [208, 209]
Postoperative Imaging
Patients should receive an MRI with and without contrast within 24-48 hours after surgery to verify the extent of resection and to establish a baseline for subsequent interventions. [58, 59] The scan should include diffusion-weighted sequences for detection of perioperative ischemia. [189]
Recurrence
Most glioblastomas recur in and around the original tumor bed, but contralateral and distant recurrences are not uncommon, especially with lesions near the corpus callosum. [210] The indications for reoperation after initial treatment are not firmly established, and while there is some evidence that gross total resection of recurrent glioblastoma is associated with improved outcomes, [28, 29] no randomized clinical trials have been conducted to investigate the survival benefit of surgery following recurrence. [59]
Reoperation is generally considered in the face of a life-threatening recurrent mass, particularly if radionecrosis rather than recurrent tumor is suspected as the cause of clinical and radiographic deterioration. Positron emission tomography (PET) scans and magnetic resonance (MR) spectroscopy have proven somewhat useful in discriminating between those entities. [163, 165, 166, 167, 168] Young age, relatively high KPS score, prolonged interval since the previous operation (ie, > 6 months), and complete resection of contrast-enhancing tumor on reoperation are all associated with a better prognosis. [211, 28, 29]
Medical Care
In a 2022 update to its evidence-based clinical practice guideline on cytotoxic chemotherapy in adults with progressive glioblastoma, the Congress of Neurological Surgeons makes the following level III recommendations [212] :
-
Adult patients may benefit from treatment with TMZ, especially those whose disease progresses after a TMZ-free interval of more than 5 months
-
Fotemustine is recommended for use in elderly patients with methylated MGMT promoter status.
-
The use of tumor treating field (TTF) with other chemotherapy may be considered.
-
Combination therapy with TMZ and other cytotoxic agents (eg, nitrosourea, cisplatin, electrohyperthermia, tamoxifen) is not recommended as stand-alone therapy.
-
Other cytotoxic therapies, such as perillyl alcohol or ketogenic diet, are not recommended as stand-alone therapy.
-
Other chemotherapeutic agents, such as platinum compounds and topoisomerase inhibitors, are not recommended.
-
Oncolytic virotherapy is not recommended.
The level II recommendations in the previous guidelines have been removed, as there is insufficient evidence to support suggestions about the use of in situ chemotherapy and nitrosureas following widespread implementation of the Stupp regimen. [46, 212]
According to a 2020 consensus review by the Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO), standard-of-care therapy for newly diagnosed glioblastoma in adults begins with maximal safe surgical resection. [58] In patients ages 18-70 with good functional status, regardless of MGMT promoter methylation, options for subsequent therapy are as follows:
-
Clinical trial participation
-
Radiotherapy for 6 weeks and concurrent TMZ, followed by six cycles of TMZ with or without tumor-treating fields
-
In addition to the above, patients with MGMT methylated tumors may receive 6 weeks of radiotherapy plus six cycles of lomustine and TMZ, with or without tumor-treating fields
For patients ages 65-70, or those with poor functional status, options in those able to tolerate multimodality therapy are as follows:
-
Radiotherapy for 6 weeks plus concurrent TMZ, followed by six cycles of TMZ with or without tumor-treating fields
-
Hypofractionated (or 6 wks) radiotherapy plus concurrent TMZ followed by six cycles of TMZ with or without tumor-treating fields
For patients ages 65-70, or those with poor functional status who are unable to tolerate multimodality therapy, therapeutic options are as follows:
-
MGMT methylated tumor: TMZ monotherapy, with or without tumor-treating fields
-
MGMT unmethylated tumor: Hypofractionated (or 6 wks) radiotherapy
-
Hospice/best supportive care
Anticonvulsant medications are usually maintained, and levels are checked intermittently. Steroids are tapered to lower doses for radiation therapy and then tapered further if possible. While taking steroids, patients should be maintained on an antiulcer agent.
Radiation therapy
The goal of radiation therapy (RT) for glioblastoma is to improve local control and survival without inducing neurotoxicity (ie, radionecrosis). RT has been an important therapeutic modality since the 1990s, when early clinical trials showed that RT delays neurologic deterioration and increases survival. [32, 33] Subsequent studies confirmed those early results and have shown further that the addition of RT to surgery alone or to surgery with chemotherapy prolongs survival in glioblastoma patients from 3-4 months to 7-12 months. [31, 34]
Most current guidelines recommend starting RT within 3-5 weeks after surgery and administering 50-60 Gy in 30 daily fractions of 1.8-2.0 Gy for 6 weeks in combination with TMZ. [213, 214] These recommendations are based on dose-response relationship data demonstrating that total doses < 45 Gy are associated with a median survival of 13 weeks while a total dose of 60 Gy is associated with a median survival of 42 weeks. Investigations of other doses and dosing schedules have shown that there is no indication for doses > 60 Gy, [215] but hypofractionated RT with a higher dose per fraction and a lower total dose (eg, 40 Gy delivered in 15 fractions of 2.67 Gy over 3 weeks) results in similar survival outcomes in patients older than 65 years and in those with a KPS score < 70. [216]
Furthermore, in patients with poor clinical factors other than advanced age (eg, postoperative neurologic complications, high tumor burden, unresectable or multifocal lesions, low treatment compliance due to social factors, rapidly progressive disease), hypofractionated RT (ie, 40-50 Gy in 15 fractions) combined with TMZ produced results comparable to those seen with standard fractionation. [217] Even smaller total doses (eg, 34 Gy in 10 fractions of 3.4 Gy or 25 Gy in 5 fractions of 5 Gy), may be appropriate in extremely frail patients. [43]
It is very important to limit exposure of critical neural structures to RT, and some clinical and research groups have modified the standard approach to minimize radionecrosis in the brainstem, cervical cord, cochlea, temporal lobes, hippocampi, and ophthalmic and optic structures. [218] However, despite advanced imaging and ongoing research efforts, there is no consensus on the optimal RT volume and margin expansions when these structures are involved. [218, 219]
Stereotactic biopsy followed by RT may be considered in certain circumstances. These include patients with a tumor located in an eloquent area of the brain, patients whose tumors have minimal mass effect, and patients whose poor medical condition precludes general anesthesia. Median survival after stereotactic biopsy and RT is reported to be from 27-47 weeks. [220]
The responsiveness of glioblastoma to RT varies. In many instances, RT can induce a phase of remission, often marked with stability or regression of neurologic deficits as well as diminution in the size of the contrast-enhancing mass. Unfortunately, any period of response is short-lived because the tumor typically recurs within 1 year, resulting in further clinical deterioration and the appearance of an expansile region of contrast enhancement. [221, 222] Early research on tumor recurrence following whole-brain RT found that the great majority (78-90%) of tumors recur within 2 cm of the original site while just 5-6% of patients develop multifocal recurrence, supporting the use of focal RT. [223, 224] Radiosensitizers, such as newer chemotherapeutic agents, [37] targeted molecular agents, [38, 39] and antiangiogenic agents [39] may increase the therapeutic effect of RT and delay recurrence. [225]
Delivery of external beam radiation therapy (EBRT) typically requires a waiting period of 3-5 weeks after tumor resection to allow for wound healing and recovery, and tumor regrowth may occur during that time. Interstitial brachytherapy, in which radioactive seeds are placed intraoperatively after tumor resection, allows immediate initiation of RT. [36] GammaTile, a brachytherapy device comprising cesium 131 (131Cs)–emitting seeds embedded in a resorbable collagen-based carrier tile, received FDA approval in 2019 for treatment of recurrent brain tumors; in 2020, approval was extended to include newly diagnosed brain tumors. Tumor cells more than 5-8 mm distant from implantation site are unlikely to benefit from interstitial brachytherapy. [226]
RT for recurrent glioblastoma is controversial, though some studies have suggested a benefit from stereotactic radiosurgery or fractionated stereotactic reirradiation. [227, 228, 229] In adult patients with progressive glioblastoma, American Association of Neurological Surgeons/Congress of Neurological Surgeons (AANS/CNS) guidelines recommend that when the target tumor is amenable to additional RT, re-irradiation should be performed to improve local tumor control and maintain or improve the patient’s neurologic status and quality of life prior to further tumor progression. [41] This re-irradiation may take the form of conventional fractionation RT, fractionated radiosurgery, or single-fraction radiosurgery. [188, 41] Fleischmann et al reported that in patients undergoing re-irradiation for recurrent glioblastoma, concomitant treatment with bevacizumab significantly reduced the rate of radiation toxicity in both the short and long term. [230]
Chemotherapy – Antineoplastic agents
Temozolomide
TMZ is an orally active alkylating agent approved by the FDA in 2005 for newly diagnosed glioblastoma, maintenance therapy, and recurrent glioblastoma. For adults < 70 years in good general and neurologic condition with newly diagnosed glioblastoma, standard first-line chemotherapy consists of TMZ (75 mg/m2 daily x 6 wk) during rRT followed by six further cycles of maintenance TMZ (150-200 mg/m2 on days 1-5 every 28 days). [31]
While TMZ is generally well tolerated, common toxicities include nausea and myelosuppression, especially thrombocytopenia and neutropenia; those occur more commonly during the adjuvant therapy period. [59] Increasing the dose of TMZ or extending the duration of chemotherapy beyond six cycles has no benefit, [231] and higher doses are associated with greater toxicity and worse quality of life. [59]
Compared with RT alone, adjuvant and concomitant TMZ with RT is associated with significantly longer median progression-free survival (6.9 vs. 5 mo) and overall survival (14.6 vs. 12.1 mo), and greater likelihood of being alive in 2 years (26% vs 10%). [45, 31]
MGMT (O6-methylguanine-DNA methyltransferase) is a DNA repair enzyme that contributes to TMZ resistance. Methylation of the MGMT promoter, found in approximately 45% of glioblastomas, results in an epigenetic silencing of the MGMT gene, decreasing the tumor cell's capacity for DNA repair and increasing susceptibility to TMZ. [232] Note the following:
-
In older patients, MGMT promoter methylation is a favorable prognostic factor and predicts response to TMZ. When treated with TMZ, patients with MGMT promoter methylation had median survival of 21.7 months, versus 12.7 months in those without MGMT promoter methylation, and 2-year survival rates were 46% versus 13.8%, respectively. [43]
-
MGMT promoter methylation status may help guide treatment decisions. In particular, elderly patients, who are at greater risk of toxicity from combined RT and chemotherapy, might be treated with RT alone if their tumors lack MGMT methylation (and hence are less likely to respond to TMZ) or be treated with TMZ alone if MGMT promoter methylation is present. [43, 232]
In a phase III clinical trial, the addition of tumor treating fields (TTF)—which disrupt cellular proliferation of tumor cells via low intensity, intermediate frequency (200 kHZ), alternating electric fields—to adjuvant TMZ extended progression-free survival by 2.7 months and overall survival by 4.9 months without reducing quality of life. [233]
Temozolomide plus bevacizumab
In two phase III clinical trials, the addition of bevacizumab—an antiangiogenic monoclonal antibody that targets VEGF—to adjuvant TMZ prolonged progression-free survival but not overall survival. [94, 234] Due to the uncertain clinical significance of this finding as well as increased toxicity (eg, early cognitive decline), bevacizumab has not been approved for the treatment of newly diagnosed glioblastoma. However, it may be useful in patients with large tumors who are highly symptomatic cannot tolerate radiotherapy. [23]
Temozolomide plus lomustine
In a small phase III clinical trial, the addition of lomustine to adjuvant TMZ following RT in newly diagnosed MGMT promoter methylated glioblastoma increased median overall survival from 31.4 months to 48.1 months. [235] However, because lomustine did not increase progression free survival, was associated with greater hematologic toxicity, and might preclude later use of lomustine (which is standard of care at recurrence), the role of lomustine in newly diagnosed glioblastoma remains unclear.
Chemotherapy for recurrent glioblastoma
Chemotherapy for recurrent glioblastoma provides modest benefit at best. Agents from several classes are used. According to the National Comprehensive Cancer Network, preferred agents include the following [22] :
-
Bevacizumab
-
TeMZ
-
Lomustine or carmustine
-
PCV (procarbazine, lomustine [CCNU], vincristine)
-
Regorafenib
Carmustine
Approved by the FDA in 2002 for the treatment of newly diagnosed and recurrent glioblastoma, biodegradable polifeprosan 20 with carmustine polymer wafers (Gliadel) are placed on the surface of the resected tumor bed at the time of surgery and then slowly degrade to release carmustine directly into the brain, thereby bypassing the blood-brain barrier. [236, 237] In a large phase III trial, carmustine wafers were shown to produce a modest increase in median survival over placebo (13.8 vs 11.6 months) but were associated with increased rates of cerebrospinal fluid leak and increased intracranial pressure secondary to edema and mass effect. [236, 238]
Similarly, in a randomized clinical trial of 222 patients, carmustine wafers increased 6-month survival from 36% to 56% over placebo but were associated with serious intracranial infections. [237, 239] At present, carmustine wafers are used only sporadically, partially because of these safety and tolerability issues but also because much of the efficacy data stem from the pre-TMZ era and use of this treatment may preclude patients from enrolling in clinical trials. [58]
Bevacizumab
Bevacizumab, an anti-angiogenic monoclonal antibody against vascular endothelial growth factor (VEGF), was approved by the FDA for recurrent glioblastoma in 2009 based on early phase II clinical data showing improved progression-free survival, though there was no improvement in overall survival. [240, 241, 242] A subsequent phase III clinical trial demonstrated that bevacizumab combined with lomustine improves progression-free survival more than lomustine alone (4.2 vs 1.5 mos), but there was still no benefit in overall survival. [243]
Similarly, bevacizumab combined with irinotecan was found to improve survival over bevacizumab alone. [244] When compared with TMZ alone, the bevacizumab-irinotecan combination also increased 6-month survival from 21% to 46%. [245, 246] Furthermore, the anti-angiogenic effect of bevacizumab reduces peritumoral edema, lowering the necessary corticosteroid dose and thus improving quality of life. [244, 247]
A population-based analysis of 5607 adult patients with glioblastoma in the SEER (Surveillance Epidemiology and End Results) database also found that bevacizumab therapy may improve survival. In the study, glioblastoma patients who died in 2010 (after the FDA approved bevacizumab for this condition) had survived significantly longer than those who died of the disease in 2008. Median survival was 8 months for patients who died in 2006, 7 months in 2008, and 9 months in 2010. This difference in survival was highly significant between 2008 (pre-bevacizumab) and 2010 (post-bevacizumab). The survival difference was unlikely due to improvements in supportive care, which did not vary significantly between those who died in 2006 and patients who died 2 years later in 2008. [248, 249]
Electric-field therapy
Tumor-treating fields (also known as alternating electric field therapy) is a noninvasive modality that involves the transcutaneous delivery of low-intensity, intermediate-frequency (200 kHZ), alternating electric fields that exert biophysical force on charged and polarizable molecules known as dipoles. This modality targets dividing cells in glioblastoma in several ways, including interference with the mitotic apparatus, DNA repair, and cell permeability. [250] Normal cells are generally not harmed. [251] The tumor-treating fields are generated via electrodes placed directly on the scalp. To target the tumor, array placement is based on the individual patient's magnetic resonance imaging results. [252]
The Optune tumor-treating field device, also known as the NovoTTF-100A System, was initially approved in 2011 for use in glioblastoma that had recurred or progressed after treatment. In 2015, the FDA expanded approval to include use of the device in conjunction with TMZ chemotherapy in the first-line setting. Approval was based on an open-label, randomized phase III trial in 700 patients in which median overall survival was 19.4 months with use of the device plus TMZ, versus 16.6 months with TMZ alone. [252] Soon after, a randomized, open-label trial in 695 patients with glioblastoma demonstrated that the addition of tumor-treating fields to treatment with TMZ improved median progression-free survival from 4.0 months to 6.7 months (P < 0.001) and median overall survival from 16.0 months to 20.9 months (P < 0.001) without compromising health-related quality of life. [233, 253, 254]
Supportive Care
Common complications of glioblastoma that may require supportive care include the following:
-
Vasogenic brain edema
-
Seizures
-
Venous thromboembolism (VTE)
Vasogenic edema
Brain edema can cause focal neurologic deficits and, by increasing intracranial pressure (ICP), produce headache, nausea, and vomiting. Corticosteroids are used to treat patients with symptoms from peritumoral vasogenic edema. Dexamethasone is the steroid of choice for these patients because of its potency, long half-life, and high brain penetrance. There is no standard regimen for steroid use in this setting, so dosing must be individualized. Most patients respond to low doses of dexamethasone (eg, 4-16 mg/day, given in 1-2 doses). [58, 255, 256]
Because of the many adverse effects of steroids, which worsen with increased dose and duration of treatment, dexamethasone should generally be used at the lowest effective dose and for the shortest period. Patients on high-dose steroids should receive concomitant gastric protection (eg, with an H2 antagonist), and those on long-term treatment (≥20 mg prednisone equivalents daily for ≥1 month) should be considered for prophylaxis against osteoporosis and Pneumocystis jirovecii pneumonia. [58]
Several studies have reported that, in addition to reducing brain edema, dexamethasone may exert an antitumoral effect by inhibiting proliferation and migration of glioblastoma cells. In contrast, other studies have reported that dexamethasone may enhance the aggressiveness of glioblastomas. These contradictory results may reflect the different actions of dexamethasone on glioblastomas with different gene expression profiles. In the future, precision medicine may address this by combining glucocorticoids with agents that inhibit the unwanted signaling pathways activated by glucocorticoids. [257, 258]
In patients at risk of herniation, ICP can be reduced emergently with mannitol and hypertonic saline, diuretics, and fluid restriction together with elevation of the head of the bed and hyperventilation. For long-term control of brain edema and treatment of steroid-refractory cases, use of antiangiogenic agents such as bevacizumab has been proposed. [255] Preliminary studies have provided support for the notion that bevacizumab can effectively reduce brain tumor-related steroid-refractory edema. [259, 260]
Seizures
Seizures are one of the most common presenting symptoms of glioblastoma, and roughly half (40-60%) of patients with GBM experience seizures over the course of the disease. [5, 261] Seizures often respond to treatment of the tumor (ie, surgical resection, radiotherapy, chemotherapy). When antiepileptic drugs (AEDs) are used, newer agents such as levetiracetam are usually administered at the lowest dose possible for seizure control in order to avoid adverse effects and minimize drug-drug interactions. [58, 255] Notably, a recent retrospective study found that preoperative seizures and levetiracetam administration were associated with a significant survival advantage in patients with glioblastoma. [262]
Prolonged primary AED prophylaxis (ie, in patients who have never had a seizure) is generally not recommended. Similarly, little evidence supports the use of AEDs to prevent postoperative seizures in glioblastoma patients who have never had a seizure; however, if AEDs are used in that setting, they should be tapered 1-2 weeks postoperatively. [58, 255]
In patients who remain seizure-free while on AED therapy, deciding when to discontinue the drug can present a clinical challenge. At minimum, a period of 1 year without seizures and with clinical and radiologic disease stability could be appropriate before considering withdrawal of AED treatment. [255]
Venous thromboembolism
Approximately 20% of glioblastoma patients experience VTE in the year following surgical resection. [58] This is due to multiple factors, including increased activation of clotting factors and thrombin by glioblastoma, the need for surgical intervention, and high rates of impaired limb mobility. [263, 264]
Prevention and treatment of VTE in these patients is complicated by their increased risk for intracranial hemorrhage (ICH). Therapeutic anticoagulation may increase risk of ICH in patients with primary brain tumors, but lack of long-term anticoagulation has been associated with an increased risk of recurrent VTE in patients with glioblastoma. [265] American Society of Clinical Oncology (ASCO) guidelines recommend anticoagulation for patients with primary brain malignancies and an established VTE, although uncertainties remain about the choice of anticoagulant and selection of patients most likely to benefit, due to limited data on this population. [266]
For cancer patients generally, ASCO guidelines recommend that those undergoing major surgery receive VTE prophylaxis with either unfractionated heparin or low molecular weight heparin (LMWH) unless contraindicated (eg, because of active bleeding or high bleeding risk). [266] In patients with systemic cancer, prophylaxis is started preoperatively; because of the risk of ICH, however, prophylaxis in glioblastoma patients is started within 24 hours after surgery. [255] Prophylaxis is continued for at least 7 to 10 days postoperatively. [266] Data from a retrospective matched cohort study found no statistically significant difference in the cumulative incidence of ICH in high-grade glioma patients in the LMWH cohort versus the cohort not receiving anticoagulation therapy. [267]
ASCO guidelines include direct oral anticoagulants (DOACs) as an option for VTE prophylaxis and treatment but note an increased risk of major bleeding. [266] However, a retrospective study by Carney et al found that in patients with primary brain tumors, the incidence of major hemorrhage was significantly lower with use of DOACs compared with LMWH. These authors concluded that DOACs are a reasonable option for treatment of VTE in this population. [268]
Consultations
To develop a coordinated treatment strategy, patients with glioblastomas should be evaluated by a team of specialists that includes a neurologist, neurosurgeon, neurooncologist, and radiation oncologist.
Investigational Approaches
The limited efficacy of current therapeutic options for glioblastoma (GBM) has prompted research into alternative approaches. Therapy modalities under investigation include the following: [58, 269]
-
Targeted molecular therapies
-
Immunotherapy (eg, vaccines, checkpoint inhibitors, oncolytic viruses) [270]
-
Nanomedicines that can cross the blood-brain barrier [271]
-
Stem cells [272]
-
Cannabinoids [273]
Genotyping of brain tumors may have applications in stratifying patients for clinical trials of various novel therapies. In about 50% of patients with gliomas, circulating tumor DNA can be sequenced from cerebrospinal fluid, allowing genotyping of the tumor without the need for brain re-biopsy. [159, 160, 161]
Vaccine therapy
Vaccines being studied for treatment of glioblastoma include modified polio vaccine, cytomegalovirus (CMV) vaccine, and autologous tumor lysate-loaded dendritic cell vaccine.
Modified polio vaccine therapy
The poliovirus receptor CD155 is broadly upregulated on the surface of malignant solid tumors, and a preliminary study of intratumoral infusion of a modified poliovirus vaccine has demonstrated benefit in some cases of recurrent malignant glioma. In a dose-finding and toxicity study, 61 patients with recurrent supratentorial WHO grade IV malignant glioma received seven doses of a live attenuated poliovirus type 1 vaccine with its cognate internal ribosome entry site replaced with that of human rhinovirus type 2. The recombinant nonpathogenic polio–rhinovirus chimera was infused into the glioma via an implanted catheter. In contrast to overall survival rates in a historical control group, which declined steadily to 14% at 24 months and 4% at 36 months, overall survival in the study patients stabilized at 21% at 24 months and remained at that rate through 36 months. [277]
Adverse events that affected more than 20% of the study patients in the dose-expansion phase included headache (52%), hemiparesis (50%), seizure (45%), dysphasia (28%), and cognitive disturbance (25%). [277] Notably, secondary analysis revealed that very low tumor mutational burden was associated with long-term survival in this recurrent GBM recombinant poliovirus trial. [278]
Cytomegalovirus vaccine
Approximately 90% of glioblastomas express CMV proteins, and Batich et al have reported benefit with a dendritic cell vaccine targeting CMV antigen pp65, using CMV as a proxy for glioblastoma. [279] Patients are first treated with dose-intensified temozolomide, as the temozolomide induces lymphopenia, which provides an opportunity to retrain the immune system.
In the study by Batich et al, 11 patients with newly diagnosed glioblastoma received temozolomide, 100 mg/m2/d × 21 days per cycle, and at least three pp65-directed vaccines admixed with granulocyte-macrophage colony-stimulating factor on day 23 ± 1 of each cycle. Despite increased proportions of regulatory T cells (Tregs), median progression-free survival was 25.3 months and overall survival was 41.1 months; three patients remained progression-free more than 7 years after diagnosis. [279]
Autologous tumor lysate-loaded dendritic cell vaccine
Dendritic cells (DCs) initiate an immune response by capturing antigens from their environment, processing them, and presenting them to naive T cells in lymphoid tissues. For creation of an autologous vaccine, monocytes are collected from the patient by leukapheresis for DC culture, and the DCs are then exposed in vitro to lysate of the patient’s tumor. This ensures that the vaccine targets the full repertoire of antigens present on the patient’s tumor. On injection into the patient, the dendritic cells present tumor antigens to the immune system, prime T cells, and mobilize antitumor responses. [280, 281]
In an international phase III trial, autologous tumor lysate-loaded dendritic cell vaccination (DCVax-L) resulted in clinically meaningful and statistically significant improvement in overall survival for patients with both newly diagnosed and recurrent glioblastoma, compared with standard of care. The survival benefit of DCVax-L was larger in patient groups that generally fare worse with standard of care, including older patients, patients with substantial residual tumor, and patients with recurrent disease. [281]
For study patients with newly diagnosed GBM, median overall survival for the 232 patients receiving DCVax-L was 19.3 months from randomization (22.4 months from surgery) versus 16.5 months from randomization in control patients (hazard ratio [HR] = 0.80; 98% confidence interval [CI], 0.00-0.94; P = 0.002). Survival at 60 months was 13.0% vs 5.7%, respectively. For the 64 patients with recurrent GBM receiving DCVax-L, median overall survival was 13.2 months from relapse versus 7.8 months in control patients (HR, 0.58; 98% CI, 0.00-0.76; P < 0.001); survival at 30 months after recurrence was 11.1% vs 5.1%, respectively. [281]
Tyrosine kinase inhibitors
A small proportion of glioblastomas responds to the tyrosine kinase inhibitors gefitinib and erlotinib. The simultaneous presence in glioblastoma cells of mutant EGFR (EGFRvIII) and PTEN is associated with responsiveness to tyrosine kinase inhibitors, whereas increased p-akt is associated with a decreased effect. [282, 283, 284] Other targets include PDGFR, VEGFR, mTOR, farnesyltransferase, and PI3K.
Checkpoint inhibitor therapy
In preclinical studies, inhibitors of programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) have shown some potential for treatment of glioblastoma. In clinical studies, however, anti-PD-1/PD-L1 monotherapy has shown few satisfactory results. Efficacy may be better in certain patient subgroups (eg, those with higher tumor mutation burden, higher microsatellite instability, mismatch repair system deficiency, germline POLE mutation). Neoadjuvant checkpoint inhibitor therapy has shown promise. [285]
CheckMate 143, a phase 3 randomized clinical trial, compared overall survival (OS) in 369 patients with recurrent glioblastoma treated with either bevacizumab or the PD-L1 inhibitor nivolumab. The 12-month OS was 42% in both groups. The objective response rate was higher with bevacizumab than with nivolumab (23.1% versus 7.8%, respectively). The rates of grade 3/4 treatment-related adverse events were similar in the two groups. [286]
Interestingly, secondary analysis of IDH-wildtype participants treated with immune checkpoint inhibitors found that very low tumor mutational burden correlated with durable, long-term survival in a subset of recurrent GBM patients. [278, 287]
Drug delivery systems
A major hindrance to the use of chemotherapeutic agents for brain tumors is that the blood-brain barrier effectively excludes many agents from the central nervous system. This has inspired the development of novel methods of intracranial drug delivery to deliver higher concentrations of chemotherapeutic agents to the tumor cells while avoiding the adverse systemic effects of these medications.
Pressure-driven infusion of therapeutic agents through an intracranial catheter, also known as convection-enhanced delivery (CED), has the advantage of delivering drugs along a pressure gradient rather than by simple diffusion. CED has been used to deliver both conventional chemotherapy drugs (eg, paclitaxel, topotecan) and investigational agents (eg, interleukin-4–Pseudomonas exotoxin fusion protein). Early preclinical and clinical studies using CED showed that it is safe but suggested it is only somewhat effective and has substantial technical limitaitons. [288]
Seeking to overcome these barriers, in a recent phase 1b clinical trial, Spinazzi et al engineered a subcutaneously implanted catheter-pump system capable of repeated, chronic CED of topotecan into the brain and tested its safety and biological effects in five patients with recurrent glioblastoma. They found that chronic CED of topotecan was well tolerated without substantial complications and significantly reduced proliferating tumor cells in all five patients, suggesting that CED may be an effective drug delivery approach in glioblastoma. [289]
Numerous research teams are exploring the use of focused ultrasound to noninvasively open the blood-brain barrier and enable relatively large agents into the brain parenchyma. In short, focused ultrasound induces microbubble oscillations that mechanically disrupt the endothelial tight junctions that contribute to the blood-brain barrier, thus allowing the delivery of therapeutics such as chemotherapies, targeted drug therapies, or immunotherapies into the brain parenchyma. While preclinical models have produced encouraging data, clinical trials exploring the use of focused ultrasound are in early, exploratory stages. Thus far, clinical trials have demonstrated that the use of focused ultrasound is safe and feasible, but further studies are warranted to evaluate efficacy. [290]
Activity
While seizures may prevent patients with glioblastomas from driving, there are no universal restrictions, and activity levels are largely determined by each patient’s overall neurologic status. In many circumstances, physical therapy and/or rehabilitation are extremely beneficial. In fact, regimented exercise programs have been shown to improve motor function and cognitive functioning and reduce depression, anxiety, headaches, fatigue, and communication deficits in patients with various forms of glioma, including glioblastoma. [291] One study even found that exercise was an independent predictor of survival in patients with recurrent high-grade (grade III and IV) glioma, [292] and an ongoing clinical trial (NCT03390569) is investigating the effects of exercise regimens on progression-free survival, overall survival, and quality of life in GBM patients.
-
Axial CT scan without intravenous contrast reveals a large right temporal intra-axial mass (glioblastoma). Extensive surrounding edema is present, as demonstrated by the peritumoral hypodensity, and a moderate right-to-left midline shift can be noted. All of the radiologic studies in this article are of the same patient.
-
A T1-weighted axial MRI without intravenous contrast demonstrates a hemorrhagic multicentric glioblastoma in the right temporal lobe. Effacement of the ventricular system is present on the right, along with mild impingement of the right medial temporal lobe on the midbrain.
-
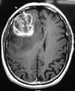
A T1-weighted axial MRI with intravenous contrast shows heterogeneous enhancement of the lesion within the right temporal lobe. The hypointensity circumscribed within the enhancement is suggestive of necrosis. This radiologic appearance is typical of a multicentric glioblastoma.
-
A T1-weighted coronal MRI with intravenous contrast demonstrates a glioblastoma within the medial temporal lobe and the stereotypical pattern of contrast enhancement.
-
A T1-weighted sagittal MRI with intravenous contrast demonstrates a glioblastoma.
-
On T2-weighted axial MRI, the tumor (glioblastoma) and surrounding white matter within the right temporal lobe show increased signal intensity compared with a healthy brain, suggesting extensive tumorigenic edema.
-
A fluid-attenuated inversion recovery (FLAIR) axial MRI. This image is similar to the T2-weighted image and demonstrates extensive edema in a patient with glioblastoma.
-
Histopathologic slide demonstrating classic glioblastoma (GBM).
-
Magnetic resonance (MR) spectroscopy signal representative of glioblastoma (GBM) demonstrating a high peak ratio of choline (CHO) to creatine (CR), a decreased N-acetylaspartate (NAA) peak, and an increased lactate (LAC) peak.
-
Hematoxylin and eosin stain of a biopsy specimen of a glioblastoma shows prominent microvascular proliferation (formation of a mulitlayered "glomeruloid tuft"). Courtesy of Wikimedia Commons [author Jensflorian, https://commons.wikimedia.org/wiki/File:Glioblastoma_endothelial_proliferations.jpg].
-
Necrosis is another histopathologic hallmark of glioblastoma. As seen here, necrotic areas often create serpentine patterns, and tumor cells form pseudopalisades around the periphery of these necrotic areas. Courtesy of Wikimedia Commons [author Jensflorian, https://commons.wikimedia.org/wiki/File:GBM_pseudopalisading_necrosis.jpg].
-
Coronal section of a glioblastoma in the left temporal lobe with typical coloration: thickened tan cortex overlying a large central region of yellowish necrosis stippled with red and brown lesions from new and old hemorrhage. Courtesy of Wikimedia Commons [author Sbrandner, https://commons.wikimedia.org/wiki/File:Glioblastoma_macro.jpg].
-
Histology section of a giant cell glioblastoma. Several bizarre, multinucleated giant cells are visible against a background of smaller tumor cells. Courtesy of Wikimedia Commons (author Jensflorian, https://commons.wikimedia.org/wiki/File:Giant_cell_glioblastoma_HE_X200.jpg].
-
Histology section of a gliosarcoma with Van Gieson’s stain highlighting connective tissue. The classic alternating pattern of gliomatous (pink) and sarcomatous (yellow-brown) tissue is evident. Courtesy of Wikimedia Commons [author Marvin 101, https://commons.wikimedia.org/wiki/File:Gliosarcoma_Histopathology_200x_EVG.jpg].
-
This glioblastoma is composed of large epithelioid cells that are immunoreactive for glial fibrillary acidic protein (GFAP) (hematoxylin and eosin, 40× original magnification). Courtesy of Roger E McLendon, MD.
-
This typical untreated glioblastoma, here with the classic "butterfly" configuration, is a necrotic hemorrhagic mass. Courtesy of Wikimedia Commons [author Rodney D McComb, MD and The Armed Forces Institute of Pathology, https://commons.wikimedia.org/wiki/File:Glioblastoma_multiforme.jpg].