Developing wide-temperature range and high-voltage aqueous MXene planar micro-supercapacitors
A recent study led by Prof. Zhong-Shuai Wu (Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS)), and Prof. Hui-Ming Cheng (Institute of Metal Research of CAS) and published in National Science Review examines aqueous MXene planar micro-supercapacitors.
MXenes, a family of 2D transition metal carbides and nitrides with over 30 species, are emerging as high-performance electrode materials. However, a MXene electrode is easily oxidized at high anodic potential in aqueous electrolytes, and its operating voltage is normally limited by the electrochemical thermodynamic stability window of water, thus resulting in small operating voltages usually less than 0.6 V, which greatly limits the energy density of MXene based MSCs (MXene-MSCs).
In addition, aqueous electrolytes freeze easily at sub-zero temperatures, leading to a sharp decline in ionic conductivity. While, at high temperatures, the structure of aqueous electrolytes is so unstable that it is difficult to retain internal water molecules because of volatility. Therefore, it is still a great challenge to develop aqueous electrolytes with high voltage and wide temperature range.
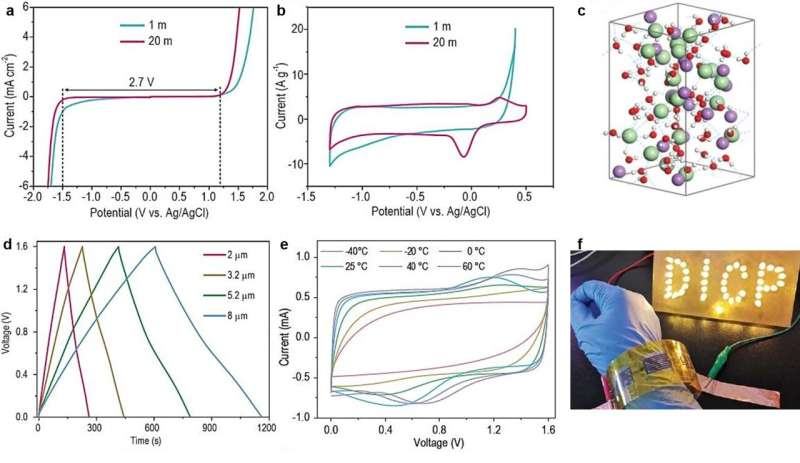
"We developed a low-cost, environment-friendly and high-concentrated LiCl aqueous electrolyte to regulate reaction kinetics of MXene (Ti3C2Tx) electrode and electrolyte, which not only broadened the operation voltage of MXene-MSCs by inhibiting oxidation at high potential, but also increased the temperature range owing to a low freezing point," said Prof. Wu.
The as-fabricated symmetric planar aqueous MXene-MSCs with the above-mentioned electrolyte achieved an operating voltage of up to 1.6 V, and energy density of up to 31.7 mWh cm-3 at room temperature.
The low freezing point (-57 °C) of high-concentrated LiCl-gel electrolyte also enabled MXene-MSCs to operate stably in a wide temperature range (-40°C to 60°C). The scalability and flexibility of MXene-MSCs makes it easy for them to be integrated into wearable microelectronics.
More information: Yuanyuan Zhu et al, Kinetic regulation of MXene with water-in-LiCl electrolyte for high-voltage micro-supercapacitors, National Science Review (2022). DOI: 10.1093/nsr/nwac024
Provided by Science China Press