Using monoclonal antibodies against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein and senescent markers, researchers provide evidence that virus-infected lung cells acquire characteristics of cellular senescence, which also leads to expression of proinflammatory cytokines.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Coronavirus disease 2019 (COVID-19) is a disease caused by the SARS-CoV-2 pathogen. It has resulted in more fatalities among the elderly and those with other diseases. Although the virus mainly infects the respiratory system, the disease can progress to various other organs along with high levels of proinflammatory cytokines, also called a cytokine storm (which can be fatal).
The viral infection is associated with DNA damage and cell fusion, which are known inducers of cellular senescence. When senescence occurs in cells, cell division and growth can cease, causing an irreversible cell-cycle arrest, changed metabolism, and proinflammatory characteristics called Senescence Associated Secretory Phenotype (SASP). Although there is little evidence linking viruses to cellular senescence, viral infection can lead to proinflammatory responses that may promote senescence.
Thus, senescence could act as a defense mechanism against viral infection and there may be increased senescence in cells that are infected and those surrounding them. In a new study published on the bioRxiv* preprint server, researchers report the results of their study of the association between SARS-CoV-2 infection and cellular senescence.
Testing SARS-CoV-2 infected lung tissues
For their study, the authors obtained lung tissue samples from 10 COVID-19 patients. They used previously developed monoclonal antibodies to the SARS-CoV-2 spike protein to test for COVID-19 in the lung cells.
The authors detected the virus in the alveolar type-II (AT2) pneumocytes of all the patients. The infected AT2 cells were sometimes large and showed strong, diffuse, or granular cytoplasmic signal in immunohistochemistry staining. The infected cells covered the alveolar wall and projected out into the airspaces, or were present in the alveolar spaces, either isolated or in clusters.
Electron microscopy confirmed the presence of the virus within the AT2 cells. At high magnification, the authors observed virions close to the endoplasmic reticulum, indicating their growth, and in the cytoplasmic vesicles indicating their release into the extracellular space.
Using reported methods for detecting senescence, the team observed that about 8 to 17% of the SARS-CoV-2 infected AT2 cells reacted with SenTraGor, a marker for senescence. They also confirmed this with another senescence marker. Sometimes these cells were clustered and expressed angiotensin-converting enzyme 2 (ACE2), another indication of SARS-CoV-2 infection mediated by ACE2.
In serial sections and co-staining analysis, the authors found proinflammatory cytokines IL-1b and IL-6, which were not seen in the non-COVID-19 tissue. This is evidence of SASP expression by senescent cells, write the authors.
The expression of these cytokines, key components of cytokine storm, suggests cellular senescence via SASP plays a role in poor clinical outcomes in some COVID-19 patients. Thus, in COVID-19 patients, senescence changes the properties of respiratory cells, causing them to produce cytokines that are released into the blood.
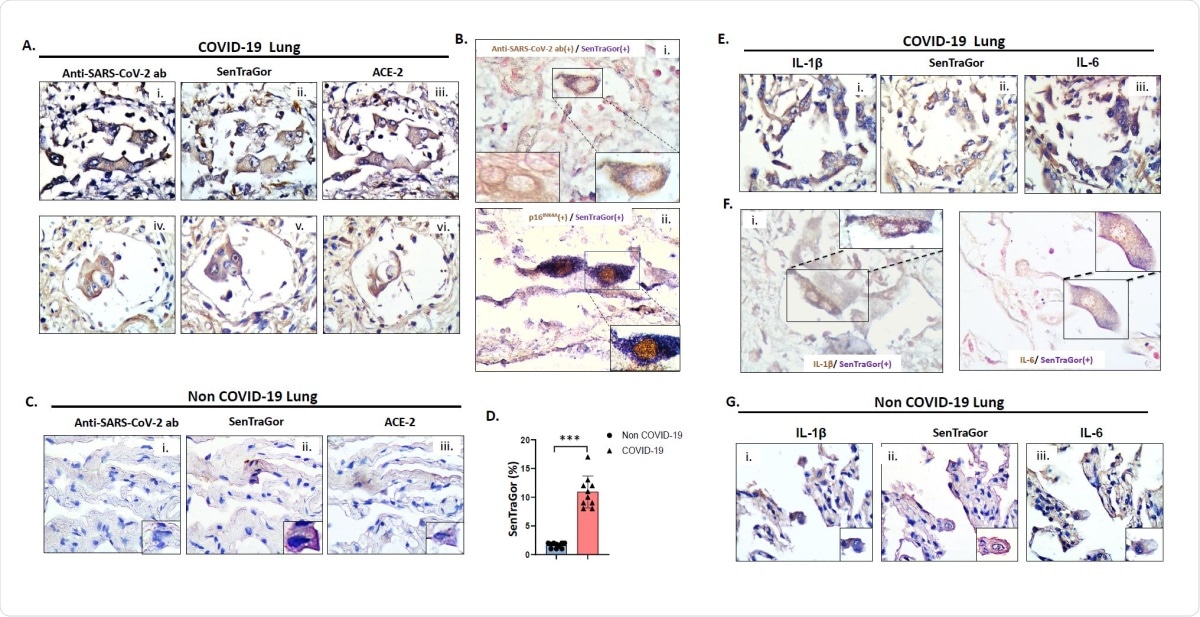
Representative images of G2 (i), SenTraGor (ii) and ACE-2 (iii) staining in serial sections of COVID-19 lung tissue. B. Representative results from double-staining experiments showing cytoplasmic localization of viral spike protein in cells that are concurrently positive with SenTraGor (senescent) (i), and nuclear p16INK4A expression in cells that are concurrently positive with SenTraGor (ii). B(i) left inset depicts an AT2 cell solely exhibiting cytoplasmic immunopositivity for the viral Spike protein. C. Representative images of G2 (i), SenTraGor (ii) and ACE-2 (iii) staining in serial sections of non COVID-19 “normal” lung parenchyma in the vicinity of a tumor (n=10, aged matched with cases presented in Fig 2A). Range of SenTraGor labeling indices: 1-2%. Morphologically, senescent AT2 cells in non COVID-19 tissue exhibit a decreased size in relation to those in COVID-19 cases. D. Graph depicting differences in SenTraGor staining between non-COVID19 and COVID19 cases, ***p<0.001 (Mann-Whitney U test). E. Representative images of Interleukin 1ß (IL-1ß) (i), SenTragor (ii) and Interleukin-6 (IL-6) (iii) in serial sections of COVID-19 lung tissue. F. Representative results from double-staining experiments showing cytoplasmic localization of IL-1ß in cells that are concurrently positive with SenTraGor (i), and IL-6 expression in cells that are concurrently positive with SenTraGor (ii). G. Representative images of IL-1ß (i), SenTraGor (ii) and IL-6 (iii) in serial sections of non-COVID-19 lung tissue (see also C). Original magnification: 400x, Insets 630x; Hematoxylin and nuclear fast red counterstain (Bi and F); DAB IHC – brown color; In co-staining SenTraGor was visualized with the BCIP/NBT chromogenic hybrid Histo-IHC reaction (dark blue perinuclear and cytoplasmic colour).
Cellular senescence could cause cytokine storm
Although the results show cellular senescence in SARS-CoV-2 infection, it is yet unknown whether senescence is already present prior to infection, making the cells more infection-prone, or if the infection causes senescence as an antiviral response.
The authors write that both cases are possible. In older patients and people with age-related diseases, senescence is expected to be high, making them vulnerable to proinflammatory response. In the samples they tested, the authors found the proportion of senescent cells was higher in patients over 73 years compared to those in younger patients.
The observed senescence could also be a response to the viral infection. The infected senescent cells could produce paracrine senescence in cells nearby. “Irrespective of the origin, both scenarios are related to SASP secretion that seems at least in part a source or even a trigger of the proinflammatory cytokines, commonly observed in the blood of COVID-19 patients,” write the authors.
Thus, the results suggest further research in the area using senotherapeutics, like senolytics, SASP modulators or inhibitors, could aid in the development of anti-SARS-CoV-2 therapeutics.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.