Detecting Nitinol Surface Inclusions
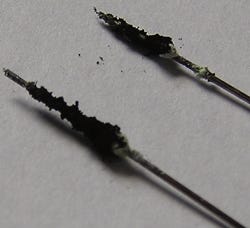
Figure 1. Strings of small oxide inclusions are elongated during the drawing process on electropolished nitinol wire surfaces.
February 1, 2010
In recent years the use of nitinol (an almost equiatomic binary intermetallic compound of nickel and titanium) has been steadily growing, particularly in the medical and dental device markets. Nitinol-based medical devices can be divided into two groups: implantables (e.g., peripheral stents, cardiovascular luminal shields, and heart valves) and surgical tools (e.g., stone and blood clot retrievers, vena cava filters, and endoscopes). Dental devices that make use of the alloy include endodontic rotary files and orthodontic archwires.
The main reasons for this growing use of nitinol arise from its combination of mechanical (pseudoplasticity and shape memory) and biocompatible properties. Nitinol can undergo reversible change between two crystal structures. In addition, it spontaneously creates titanium-rich oxide. Some research has shown that titanium-rich oxide on nitinol stents can minimize the formation of fibrin- and platelet-rich thrombus.1
There has been some effort by nitinol producers and scientists around the world to develop almost totally passive inclusion-free nitinol, with improved fatigue and corrosion resistance properties. Some of these approaches include smart anodization, acidic and basic chemical etching, heat treatment in a different gaseous atmosphere, ion implantation, cryogenic treatment, electropolishing and magnetoelectropolishing.2,3 However, these methods do not place emphasis on homogeneity and, therefore, cannot guarantee an inclusion-free surface.
|
Table I. Origin inclusions are easier to identify than chemical inclusions. Chemical analysis can often lead to errors. |
Some research has shown that about 80% of crack initiations in nitinol stents are triggered by visible inclusions.4 In response to this research, nitinol producers in Europe offer what is called extra-low-inclusion nitinol (such as that supplied by Euroflex). Nonmetallic inclusions have been frequently shown to be corrosion and crack initiation sites in superelastic nitinol.5 Surface inclusions cause stress during rotational bending and flexing (particularly for peripheral stents) and can lead to fracture.6 In addition, the inclusions and nonhomogenous adjacent matrix are thought to act as sources for corrosion and can release harmful nickel ions to surrounding living cells, which can create inflammation or stimulate intimal hyperplasia in implanted peripheral nitinol stents.7
It should be noted that there is some controversy attached to the topic of this article, in that some leading experts in the field do not agree with the description of overall nitinol surface inclusion problems or the premise of the test proposed. However, academic pursuits, particularly those of Svetlana Shabalovskaya, associate of the Ames Laboratory at Iowa State University, indicate some validity. Her research, along with others who have been researching problems of nitinol surface inclusions for the past several years, shows that inclusions are not trivial and calls for further study.
Inclusion Classifications
Nitinol inclusions are classified in two ways: by origin and chemical composition. Classification by origin gives two kind of inclusions: native (introduced during production of bulk material) and foreign (introduced during processing and finishing of detailed medical devices). Native inclusions are distributed throughout the whole volume of the material including surfaces. In contrast, foreign inclusions are strictly surface phenomena (see Table I). Classification by chemical composition is more complicated.
Because of the very small size of inclusions, chemical analysis is difficult and often leads to errors. Chemical inclusions could be broadly classified as carbides (TiC), oxides (Ti4Ni2Ox, TiO2) or intermetallic precipitates (Ni4Ti3, Ni3Ti).8,9 It is widely recognized that carbides are primarily created during vacuum induction melting (VIM), for which graphite crucible is the source of carbon. Oxides are created in larger amounts and in larger particle sizes during vacuum arc melting (VAM) than during VIM. A third process, which claims 4–10 times lower carbon content due to use of water-cooled copper crucible, is electron beam melting (EBM).10 Although these methods of nitinol production are widely used, none of them produce 100%-inclusion-free homogenous nitinol.
|
Figure 2. Nitinol wires after one week of submersion in 6% NaClO. On the left is an inclusion free surface. The right shows surface inclusions. |
It is worth mentioning that native, as well as foreign surface inclusions, undergo physical changes under mechanical production processes such as stamping and drawing. Those physical changes consist of fragmentation of inclusions under mechanical force. For example, the drawing process breaks down transverse inclusions, making them smaller as the diameter of the wire or tube reduces. Longitudinal inclusions break down and form strings of smaller inclusions that are elongated in the direction of drawing process (see Figure 1). This phenomenon carries both benefits and drawbacks.
Small-diameter wires and tubes have better fatigue resistance than larger parts because small inclusions are less likely to initiate fatigue cracks. There is also a higher probability of developing corrosion and nickel leaching sites from multiple inclusions spread over a large surface area. The high number of corrosion sites decreases the probability of stopping the corrosion process by repassivation.
Local martensitic transformation and stress concentration points are other problems that can be created by inclusions. For example, the creation of titanium carbide (TiC) inclusions drains titanium from adjacent matrices. 8, 11–13 The matrix depleted of titanium will change its martensitic transformation temperature compared with the rest of material, which can lead to unexpected voids and crack initiation.
Testing for Inclusions
The most common ways to test nitinol surfaces for inclusions require instrumental observation techniques. These techniques are transition electron microscopy, Auger electron spectroscopy with back scatter electron detection, scanning electron microscopy with energy-dispersive x-ray spectroscopy, atomic force microscopy, and x-ray diffraction (XRD). All of these techniques are expensive and time consuming. They demand highly trained operators and sophisticated equipment, and they are neither effective nor practical for mass inspection on an industrial scale. To overcome the inability to check every nitinol-based implantable device (especially peripheral stents) for surface inclusions before implantation, a chemical test has been developed, which is currently pending U.S. patent. The chemical test is simple and resembles the old but still-used ASTM standard A262 copper-copper sulfate test for testing stainless steel for ferrite formation. The inclusions test uses 6% sodium hypochlorite (NaClO) as a reagent and requires 15 minutes of immersion and observation to conclude presence or absence of surface inclusions.
|
Figure 3. Inclusion-free nitinol wire after six months of exposure to a 6% solution of NaClO. |
As long as the interatomic bonds between nickel and titanium in nitinol are intact, the intermetallic compound stays totally inert when exposed to aqueous solution of NaClO. But when those bonds are broken by precipitated inclusions, the nitinol becomes prone to corrosion by NaClO. The mechanism of this corrosion arises from the aggressiveness of NaClO toward nickel. When nickel is exposed to NaClO, a chemical reaction starts immediately. It continues until all nickel is dissolved or NaClO is spent according to the chemical reaction as follows:
2Ni + 3NaClO + 3H2O → 2Ni(OH)3 ↓ + 3NaCl
The visual sign of this reaction is black flocculent precipitate of Ni(OH)3 ↓.
When homogenous, inclusion-free nitinol surface is exposed to NaClO, nothing happens (see Figure 2 and Figure 3). Spontaneously (by ambient atmosphere) or artificially (by electropolishing process for example) created TiO2 efficiently protects nitinol against corrosion. Even if the nitinol is broken when submerged in NaClO, if the broken surfaces do not contain inclusions, corrosion does not start. Because freshly broken inclusion-free surfaces do not possess sites enriched in nickel atoms, the NaClO spontaneously oxidizes the surface to create TiO2, which prevents corrosion. In addition, inclusion-free electropolished nitinol surfaces are not attacked even during boiling in 6% NaClO. The surface also retains the reflective quality from the electropolishing. But if nitinol possesses surface inclusions, corrosion starts almost immediately. Characteristic black flocculent oozes from the reaction site (see Figure 2, right), which is inseparable from effervescence of oxygen gas O2↓ according to the following reaction:
9NaClO + 2NiTi + 7H2O → 2Ni(OH)3↓ + 2Ti(OH)4↓ + 9NaCl + O2↑
The dissolving nickel can come from two sources. The first is from the matrix surrounding inclusion, which is enriched in nickel during the process of creating inclusion by draining titanium elements to create inclusion as TiC, for example. The other source is the inclusion itself, which is enriched in nickel (e.g., intermetallic inclusions of Ni4Ti3, Ni3Ti created during wire drawing operation). But it does not matter from which source Ni(OH)3↓ originates because both sources indicate the presence of inclusions and nonhomogeneous nitinol. Titanium hydroxide, Ti(OH)4 (white precipitate) also originates during corrosion reaction, but is masked to some extent by the black color of Ni(OH)3, although it can be seen with the naked eye. These two separate corrosion reaction products, with their distinguished colors, are indicators that nonhomogenous nitinol dissolves separately as nickel and titanium and not as an intermetallic compound (see Figure 4).
|
Figure 4. Nitinol wire corroded in places of inclusions. The white precipitate is titanium hydroxide and the black precipitate is nickel hydroxide. |
Sporadically an intermediate green compound of NiCl2 × 6H2O is formed (see Figure 5). This phenomenon is not yet fully understood, but probably depends on the chemical composition of a particular inclusion. These three precipitates (distinguished by color) originate at the inclusion sites and are released in the presence of NaClO. It is worthwhile to mention that XRD analysis of precipitate of dissolved nitinol has detected only one compound as crystalline—namely NaCl—and the rest of materials were in amorphous form. This finding is also proof that NiCl2 × 6H2O is an intermediate compound because the compound possesses crystalline structure but was not detected by XRD.
Until now, only instrumental techniques have been available to check nitinol for the presence of surface inclusions and these are used for research purposes. Those methods are complicated, expensive, and not applicable in all cases (e.g., 3-D and complicated shapes). For those reasons, they are not used as inspection tools to check nitinol surface inclusions of finished medical devices. Currently nitinol-based implantable devices such as peripheral stents undergo optical inspections (e.g., by the computer-assisted automated stent inspection system from CTR Carinthian Tech Research AG), but those techniques are not able to detect surface inclusion. They can detect defects that measure 200 µm and larger, but 200 µm is far beyond the size of an average inclusion.
|
Figure 5. Nitinol wire with intermediate corrosion product showing a green precipitate of NiCl2 × 6H2O. |
The chemical test enables analysis of both raw material and finished products. In the event of positive test results of raw material intended for production, material can be rejected before starting expensive manufacturing processes, thereby saving money and time. The postproduction test of finished products can eliminate defective products, thereby limiting the risk of serious or life-threatening problems (such as fracture of endodontic rotary files, carotid stents, heart valves, or neurovascular coils, used to treat brain aneurysm).
As mentioned earlier, original nitinol inclusions are distributed throughout the volume of material. Therefore, a negative test result of raw material does not mean that finished product surfaces are free of inclusions. During production operations mechanical, chemical, or electrochemical processes remove excess material and original inclusions could be revealed from the interior of material. Also during this time, foreign inclusions could be introduced to finished surfaces by manufacturing processes such as laser cutting and sand blasting.
Experts in the nitinol field generally agree that electropolishing is the gold standard of finishing processes for nitinol implants. Electropolished nitinol implants have shown superior results in research compared with implants finished by other processes in terms of corrosion resistance, biocompatibility, reduced nickel leaching, and fatigue resistance, except for magnetoelectropolishing.
|
Figure 6. XPS data showing the compounds of electropolished (EP) nitinol wire surfaces exposed to 6% solution of sodium hypochlorite (NaClO) for 24 hours (EP+NaClO) and magnetoelectropolished (MEP) nitinol wire surfaces exposed to 6% solution of NaClO for 24 hours (MEP+NaClO). |
The question of whether the test can damage or mitigate the properties of electropolished or magnetoelectropolished inclusion-free nitinol surfaces has been raised, but such damage is unlikely. The XPS results (see Figure 6) comparing composition of surface oxides on electropolished and magnetoelectropolished inclusion-free nitinol before and after 24 hours exposure to 6% NaClO do not show chemical composition changes.
Conclusion
The proposed test for checking nitinol surfaces for inclusions presents the possibility to inspect each implantable nitinol device and increase its safety margin. Device OEMs may benefit from adopting a policy to test each single implantable device. Doing so could increase the safety of peripheral stent or heart valve recipients. The process could eliminate devices that show a high probability of fracture or jeopardized effectiveness through activating and speeding up of the restenosis process.
Acknowledgments
The author would like to thank professors Tadeusz Hryniewicz and Krzysztof Rokosz of Politechnika Koszalinska, Division of Surface Electrochemistry, Poland for providing XPS data.
References
1. B Thierry et al., “Nitinol versus Stainless Steel Stents: Acute Thrombogenicity Study in Ex Vivo Porcine Model,” Biomaterials 23 (2002): 2997–3005.
2. AW Hassel, “Surface Treatment of NiTi for Medical Application,” Minimally Invasive Therapy and Allied Technology 13, no. 4 (2004): 240–247.
3. R Rokicki et al., “Nitinol Surface Finishing by Magnetoelectropolishing,” Transaction of Institute of Metal Finishing 86, no. 5 (2008): 280–285.
4. XM Wang, YF Wang, and ZF Yue, “Finite Element Simulation of the Influence of TiC Inclusions on the Fatigue Behavior of NiTi Shape-Memory Alloys,” Metallurgical and Materials Transactions A 36, no. 10 (October, 2005): 2615–2620.
5. G Siekmeyer et al., “The Fatigue Behavior of Different Nitinol Stent Tubes Characterized by Micro Dog-Bone Testing,” Medical Device Material IV, Proceedings 7, from the Materials and Processes for Medical Devices Conference (2007): 88–93.
6. DE Allie et al., “Nitinol Stent Fractures in the SFA,” Endovascular Today (July/August 2004): 22–34.
7. LHG Franca et al., “Update on Vascular Endoprostheses (Stents): from Experimental Studies to Clinical Practice,” Jornal Vascular Brasileirovol 7, no. 4 Porto Alegre (December 2008): 351–363.
8. DW Norwich et al., “A Study of the Effect of Diameter on the Fatigue Properties of NiTi Wire,” Journals of Materials Engineering and Performance 18, no. 5–6 (2009): 558–562.
9. S Shabalovskaya et al., “Nitinol Surfaces for Implantation,” Journal of Materials Engineering and Performance 18, no. 5–6 (2009): 470–474.
10. CTA Moreira et al., “Corrosion Behavior of Equatomic NiTi SMA in Sodium Chloride Solution,” 17°CBECMat—Congresso Brasileiro de Cencia dos Materiais, Foz do Iquacu, PR Brasil (November 15, 2006).
11. S Shabalovskaya et al., “Recent Observations of Particulates in Nitinol,” Material Science and Engineering A (2008): 431–436.
12. A Toro et al., “Characterization of Non-Metallic Inclusions in Super Elastic NiTi Tubes,” Journal of Materials Engineering and Performance 18, no. 5–6 (2009): 448–458.
13. S Shabalovskaya et al., “The Effect of Surface Particulates on the Corrosion Resistance of Nitinol Wire,” Shape Memory Superelastic Conference Asilomar (2003): 399–408.
Ryszard Rokicki is president of Electrobright (Macungie, PA).
About the Author(s)
You May Also Like
.jpg?width=700&auto=webp&quality=80&disable=upscale)



.png?width=300&auto=webp&quality=80&disable=upscale)
