How is a metal formed?
What does it mean to be a metal? How is a metal formed? These seem like textbook questions with a simple answer: Metal is characterized by free electrons that give rise to high electric conductivity. But how, exactly, is a metallic conduction band formed from originally localized electrons, and what is the corresponding microscopic picture for the material involved?
In collaboration involving scientists from the Czech Republic, the U.S. and Germany, the research team of Pavel Jungwirth from the Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences (IOCB Prague) has succeeded in mapping at the molecular level the electrolyte-to-metal transition in alkali metal—liquid ammonia solutions using a combination of photoelectron spectroscopy (PES) and electronic structure calculations. The results of their research were recently published in Science.
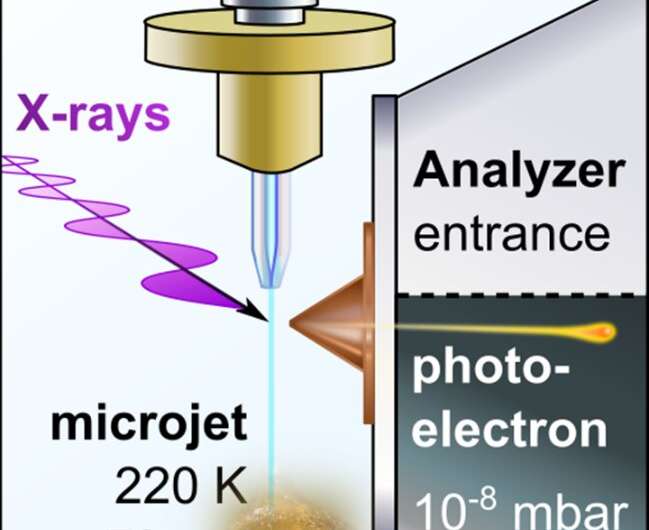
Alkali metals dissolved in liquid ammonia represent archetypal systems to explore the transition from blue electrolytes at low concentrations to bronze- or gold-colored metallic solutions (with conductivity comparable to a copper wire) with higher concentrations of excess electrons. At the same time, PES represents an ideal tool for establishing the electronic structure pertinent to this transition. As an ultra-high vacuum technique, PES was long thought to be incompatible with volatile liquids until the technique of liquid microjets was developed for water and aqueous solutions. However, it was only in 2019 that the group of Pavel Jungwirth in collaboration with colleagues at the University of Southern California and at the BESSY II synchrotron in Berlin performed the first successful PES measurements on a refrigerated polar liquid—pure liquid ammonia.
"This is what happens when you give a theory group a bit of lab space to play," says Pavel Jungwirth of the decision of the Institute's director to grant him a small laboratory.
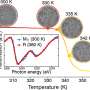
This achievement opened the door to PES studies of alkali metal—liquid ammonia systems (as reported in the present paper in Science), which map the electrolyte-to-metal transition for lithium, sodium and potassium dissolved in liquid ammonia by means of PES using soft X-ray synchrotron radiation. In this way, researchers captured for the first time the photoelectron signal of excess electrons in liquid ammonia as a peak at around 2 eV binding energy. This peak then broadens asymmetrically toward higher binding energies upon increasing the alkali metal concentration, gradually forming a conduction band with a sharp Fermi edge accompanied by plasmon peaks, both of which are fingerprints of the nascent metallic behavior.

Together with state-of-the-art electronic structure calculations, these measurements provide a detailed molecular picture of the transition from a non-metal to a metal, allowing researchers to better understand the onset of metallic behavior characterized by properties such as high electric conductivity.
"Hopefully, the present work on metallic ammonia will open the path to realizing our most 'explosive' idea: the preparation of metallic water by very carefully mixing it with alkali metals," concludes Pavel Jungwirth.
More information: Tillmann Buttersack et al. Photoelectron spectra of alkali metal–ammonia microjets: From blue electrolyte to bronze metal, Science (2020). DOI: 10.1126/science.aaz7607
Journal information: Science