In a recent study published in the journal Advanced Science, researchers developed molecularly imprinted nanoparticles (nanoMIPs) with broad-spectrum activity against lethal viruses.
 Study: Rational Development of Hypervalent Glycan Shield-Binding Nanoparticles with Broad-Spectrum Inhibition against Fatal Viruses Including SARS-CoV-2 Variants. Image Credit: Kateryna Kon / Shutterstock
Study: Rational Development of Hypervalent Glycan Shield-Binding Nanoparticles with Broad-Spectrum Inhibition against Fatal Viruses Including SARS-CoV-2 Variants. Image Credit: Kateryna Kon / Shutterstock
Viral infectious diseases are a profound threat to humans. The recent coronavirus disease 2019 (COVID-19) outbreak has severely threatened global public health, economy, and social development. Notwithstanding the development of multiple prophylactic and therapeutic strategies, the rapidly-changing viral antigenic profiles present significant challenges, as exemplified by the mutant variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Thus, broad-spectrum inhibitors of viruses are highly warranted.
Glycosylation, the universal post-translational modification, plays many crucial roles. Several viruses have evolved to use host translational machinery to modify their proteins with ‘self’ glycans, resulting in highly glycosylated viral envelopes. These glycosylated proteins shield immunogenic surfaces with a dense sheath of host-derived glycans, thereby facilitating immune escape.
Molecularly-imprinted polymers (MIPs), also known as plastic/artificial antibodies, are synthetic receptors with antibodies that mimic antibody binding through copolymerization in the presence of templates. Due to ease of preparation, storage stability, and cost efficiency, MIPs exhibit potential for different applications such as diagnosis, cancer therapy, virus recognition, and toxin neutralization. MIPs have been developed against viruses, but none with broad-spectrum activity.
The study and findings
In the present study, researchers in China developed glycan shield-binding nanoMIPs with broad and potent activity against high mannose glycan-carrying viruses. NanoMIPs were synthesized through reverse microemulsion-confined epitope-oriented surface imprinting and cladding (ROSIC) technique. Likewise, non-imprinted nanoparticles (NIPs) were also synthesized using the same procedure without templates.
The resultant nanoMIPs had a well-defined spherical morphology with a mean diameter of 39.5 nm. The specific adsorption of mannose by the nanoMIPs was significantly high. Of note, nanoMIPs had superior performance to some mannose-binding lectins. NanoMIPs exhibited little/no binding activity to non-glycosylated proteins. Each nanoMIP was capable of binding more than 50 high-mannose glycans.
The authors next studied the binding and kinetics of nanoMIPs to proteins with high-mannose glycans using biolayer interferometry. First, RNase B was used as the target protein, and the dissociation constant (Kd) was 1.3 x 10-6 M, two-to-three orders of magnitude improved relative to the Kd of mannose. The Kd for SARS-CoV-2 S1 protein was 5.3 x 10-7 M. In contrast, NIPs did not bind to RNase or SARS-CoV-2 S1.
Further, the researchers found that the binding of nanoMIPs to SARS-CoV-2 pseudovirus was enhanced by three orders of magnitude relative to the SARS-CoV-2 S1 protein, suggesting that nanoMIPs could bind virions with high avidity. Next, the authors assessed the competitive binding of nanoMIPs with angiotensin-converting enzyme 2 (ACE2) at protein and pseudovirus levels. At the protein level, ACE2 binding to SARS-CoV-2 S1 was not observed even at high ACE2 concentrations (up to 200 nM).
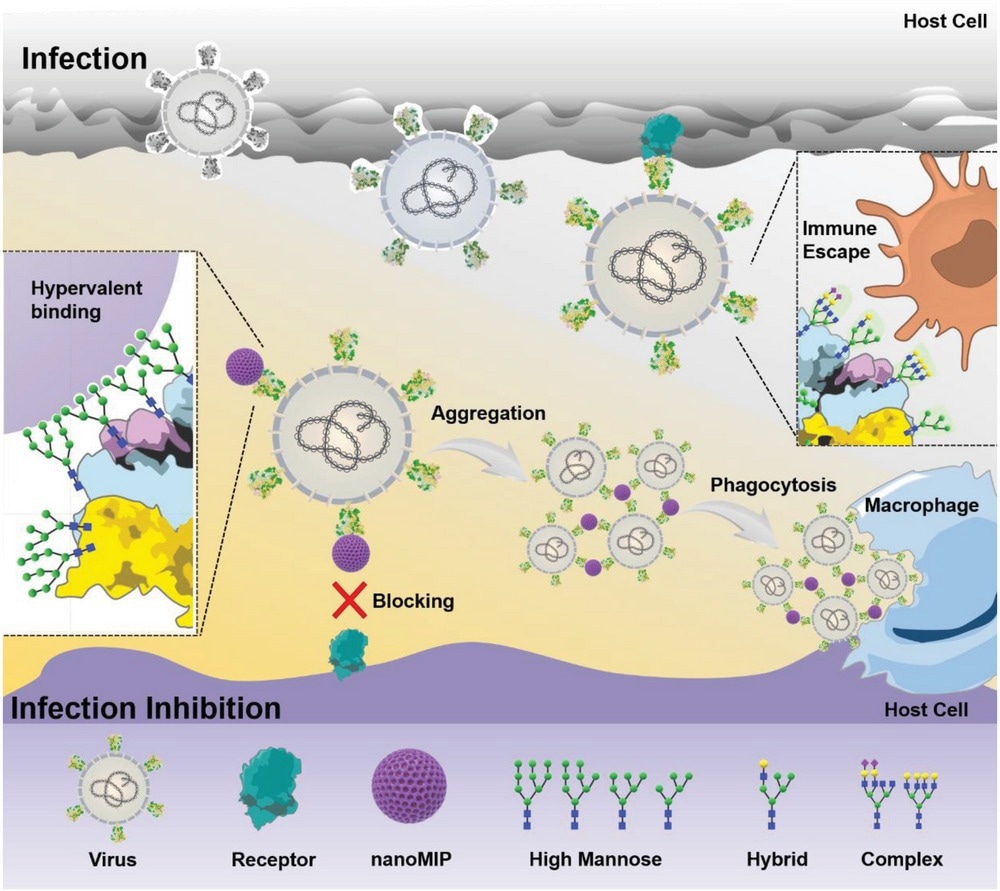
Illustration of virus inhibition by anti-high mannose nanoMIP.
At the pseudovirus level, ACE2 binding (to pseudovirus) decreased with increasing concentrations of nanoMIPs, with complete inhibition at 100 μg/ml. In a pseudovirus neutralization assay, nanoMIPs exhibited 90.2% inhibition of pseudo-viral particles of wild-type SARS-CoV-2. Similarly, high inhibition efficacy was observed with pseudoviruses of SARS-CoV-2 mutants harboring N439K, N501Y, D614G, or Δ69-70 mutations and the SARS-CoV-2 Delta and Omicron variants.
The nanoMIPs inhibited 95.5% of pseudo particles of the Lasso virus and 97.2% of HIV pseudoviruses, supporting the broad-spectrum activity of nanoMIPs against Lasso virus, HIV, and SARS-CoV-2 and its mutant variants. SARS-CoV-2 pseudoviruses treated with nanoMIPs aggregated into clusters, with only a few pseudo-particles outside the clusters. Moreover, SARS-CoV-2 pseudoviruses labeled with fluorescent markers were treated with nanoMIPs and incubated with host cells.
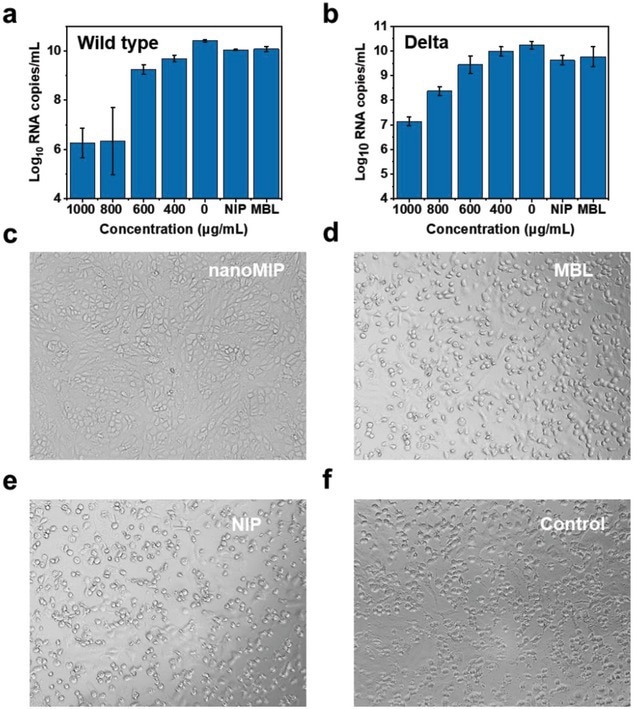
Potent inhibition of live viruses. a,b) The authentic SARS-CoV-2 virus (wild type and Delta) RNA load at 3 days post-infection from Vero cells treated with different concentrations of nanoMIP. Mean ± SD, n = 3. c–f) Cytopathic effect (CPE) images of Vero cells treated with nanoMIP (800 µg mL−1), NIP (800 µg mL−1), and MBL (10 µg mL−1) under the infection of live SARS-CoV-2 for 3 days.
Fluorescence images demonstrated that nanoMIPs could cross-link virions efficiently, aggregating around the host-cell membrane. The nanoMIP-induced aggregates of pseudoviruses were of sub-micron to micron size. Further experiments revealed that the nanoMIP-induced pseudovirus aggregates could enhance phagocytosis, activate innate immunity, and facilitate viral inactivation.
Finally, the researchers evaluated the potency of nanoMIPs to neutralize authentic wild-type SARS-CoV-2 and the Delta variant. NanoMIPs suppressed infection of Vero cells with authentic SARS-CoV-2 (wild-type and Delta variant). Viral RNA load declined with increasing nanoMIP concentrations for both wild-type and Delta variant. There was little/no cytopathic effect (CPE) with nanoMIP treatment as opposed to obvious CPE with treatment with NIPs or mannose-binding lectins.
Conclusions
In summary, the researchers developed hypervalent glycan shield-binding artificial antibodies with high potency and breadth against several viruses. The high binding avidity, steric hindrance, and rigid structure of nanoMIPs effectively blocked interactions between host cells and the viral particles. NanoMIPs induced viral aggregation by binding concomitantly to multiple virions, inhibiting viral entry into host cells and facilitating phagocytosis.
Due to both blocking and cross-linking abilities, the hypervalent nanoMIPs offered a unique strategy for potent and broad-spectrum inhibitory activity against different viruses, shifting from antigens/epitopes to glycan shields of viruses, thereby circumventing the challenges associated with viral diversity and mutations.