- Triastek's third FDA IND clearance for a 3D printed medicine
- T21, a novel potential treatment capable of colon-targeted drug delivery
NANJING, China, Nov. 21, 2022 /PRNewswire/ -- Triastek, Inc. ("Triastek") a global healthcare company pioneering 3D printing of pharmaceuticals with its proprietary technology, Melt Extrusion Deposition (MED), announced today that it has received clearance for its Investigational New Drug (IND) application from the United States Food and Drug Administration (FDA) to initiate clinical studies of the 3D printed medicine, T21, a potential treatment for ulcerative colitis.
"We are excited to receive IND clearance to begin clinical trials of this potentially transformative treatment for patients, " said Dr. Senping Cheng, founder and CEO of Triastek. "Delaying drug release and delivering oral dosage forms to the colon is challenging, so T21 offers a promising new option for patients by providing site-specific drug delivery and localized drug effect, mitigating potential side effects from systemic exposure. We look forward to advancing this treatment into the clinic and appreciate the FDA's support. "
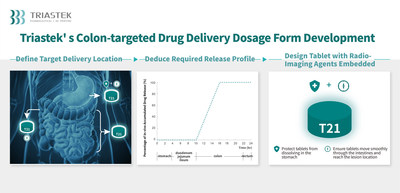
T21 is a novel potential treatment capable of colon-targeted drug delivery. With a unique 3D dosage form design, T21 can reach the targeted colon segment of the GI tract, thus permitting use of a lower drug dose than the reference listed drug, which provides systemic exposure. Made possible by 3D printing, radio-imaging agents embedded in specific components of the dosage form were employed in early formulation development to evaluate and confirm the location of drug release in the GI tract. This novel process can be utilized in the development of future drugs targeted to specific segments of the GI tract to increase the efficiency and success rate of product development. The delayed-release, colon-targeted oral tablet technology platform can not only be used for developing small molecule drugs, but for peptide drugs as well.

Triastek had already received IND clearance from the FDA for its T19 and T20 products for treatment of rheumatoid arthritis and cardiovascular and clotting disorders, respectively. Triastek continues to demonstrate the broad applicability of its MED technology through the advancement of products that solve a wide range of drug delivery challenges across multiple therapeutic areas.
About Triastek
Triastek, Inc. is a global healthcare company, developing medicines with proprietary, pioneering 3D printing technology. Triastek is dedicated to revolutionizing the development and manufacturing of pharmaceutical products and unlocking the next generation of medicine through 3D printing technology. Its state-of-the-art technology, called Melt-Extrusion Deposition (MED), facilitates the development of medicines in a layered construction to enable controlled and precise release of treatments. This optimized treatment design helps control when, where and how much medicine is released in the body, ensuring a more targeted and efficacious delivery.
About Melt-Extrusion Deposition (MED)
Melt-Extrusion Deposition (MED) 3D printing is an additive manufacturing, end-to-end technology that continuously converts powder feedstocks into softened/molten states followed by precise layer-by-layer deposition to produce objects with well-designed geometric structures.

Photo - https://mma.prnewswire.com/media/1950911/image_5017147_11354335.jpg
Photo - https://mma.prnewswire.com/media/1950912/image_5017147_11354695.jpg
Logo - https://mma.prnewswire.com/media/1859327/global_Logo_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/triastek-receives-fda-ind-clearance-for-3d-printed-medicine-for-the-treatment-of-ulcerative-colitis-301682686.html
View original content:https://www.prnewswire.co.uk/news-releases/triastek-receives-fda-ind-clearance-for-3d-printed-medicine-for-the-treatment-of-ulcerative-colitis-301682686.html


