This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Unraveling the structural dynamics of photosystem II with femtosecond X-ray crystallography
Understanding the molecular mechanisms underlying the phenomenon of photosynthesis can enable significant progress in the fields of biotechnology and renewable energy. Photosystem II (PSII), a protein complex, plays a central role in this process by catalyzing the oxidation of water and producing dioxygen using sunlight, a fundamental step in oxygenic photosynthesis. Despite extensive research, the structural dynamics of PSII during the water-splitting reaction, especially at the atomic level and on short timescales, remain largely unexplored.
Previous research has provided valuable insights into the structural changes occurring in PSII during the water-splitting reaction, focusing on microsecond to millisecond timescales. However, there has been a lack of high-resolution structural information at shorter timescales, particularly during the transitions between different states of the oxygen-evolving complex (OEC) induced by light excitation, which is essential for understanding the mechanism of water oxidation and oxygen evolution.
To address this research gap, Professor Michihiro Suga and Professor Jian-Ren Shen from the Research Institute for Interdisciplinary Science, Graduate School of Environmental, Life, Natural Science and Technology, Okayama University in Japan, utilized pump-probe serial femtosecond X-ray crystallography (TR-SFX), a technique which is known to capture ultrafast structural changes in biological macromolecules with remarkable spatial and temporal precision.
Following established protocols, PSII microcrystals were meticulously prepared and subjected to one or two laser-flashes light excitation, followed by illumination with femtosecond X-ray pulses generated by an X-ray free electron laser (XFEL).
"The process of generating microcrystals for photosystem II was time-consuming, spanning nearly five years until the findings were compiled and published," said Professor Michihiro Suga.
By exposing the crystals to laser flashes and capturing X-ray diffraction patterns at various time delays, the researchers could extensively track minor structural alterations in PSII, ranging from nanoseconds to milliseconds post-flash illumination.
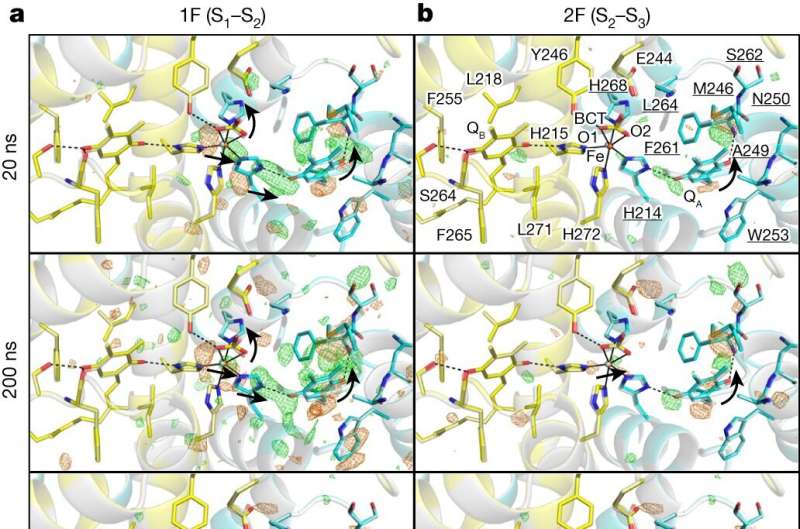
The findings, published in Nature, reveal the intricate structural dynamics of PSII during crucial transitions from the S1 to S2 and S2 to S3 states to understand pivotal events such as electron transfer, proton release, and substrate water delivery.
After exposing the crystals to laser flashes, rapid structural alterations in the YZ tyrosine residue were observed, suggesting the occurrence of fast electron and proton transfer processes.
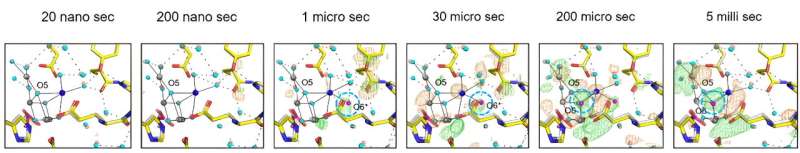
A water molecule near Glu189 of the D1 subunit was found immediately following two flashes, which subsequently transferred to a position named O6 near O5 as previously found, providing valuable insights into the origin of the oxygen atom incorporated during the water-splitting reaction.
The investigation also clarified the concerted movements of water molecules within specific channels, elucidating their crucial role in facilitating substrate water delivery and proton release. These observations shed light on the intricate interplay between the protein scaffold and water molecules, highlighting their synergistic contribution to the efficiency of the PSII's catalytic cycle.
"The findings from our research have significant implications for various fields, particularly in the design of catalysts for artificial photosynthesis. By elucidating the molecular mechanisms underlying water oxidation in PSII, we can inspire the development of synthetic catalysts capable of efficiently harnessing solar energy through artificial photosynthesis," Professor Jian-Ren Shen explains.
The researchers say that by understanding the structural dynamics of PSII, we can also inform strategies for optimizing natural photosynthetic processes in crops to enhance agricultural productivity and mitigate the effects of climate change. Adding that these findings not only deepen our understanding of the fundamental biological processes but also hold tremendous promise for addressing pressing global challenges related to energy sustainability and environmental conservation.
More information: Hongjie Li et al, Oxygen-evolving photosystem II structures during S1–S2–S3 transitions, Nature (2024). DOI: 10.1038/s41586-023-06987-5
Journal information: Nature
Provided by Okayama University