Crystalline cages that adsorb water have found an unexpected application in the field of sensors. These supramolecular structures serve as ‘touchless’ sensors, by simply detecting the humidity that naturally surrounds the skin. The sensor ‘exhibits an exceptional responsiveness to humidity changes’, explains lead author Niveen Khashab, who works at the King Abdullah University of Science and Technology in Saudi Arabia. ‘It’s a more promising avenue for touchless technology,’ she adds.
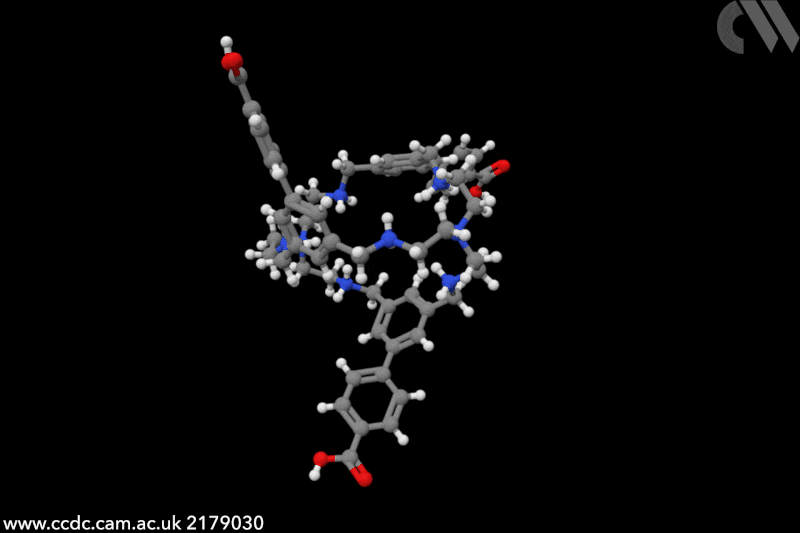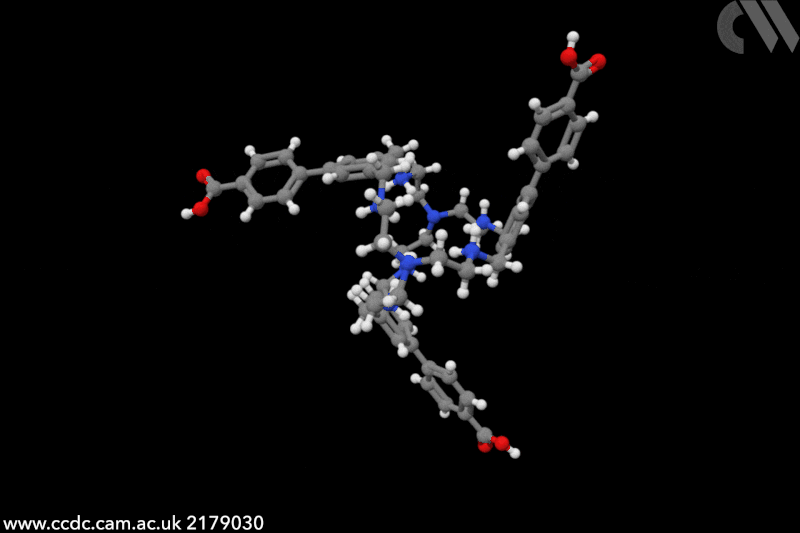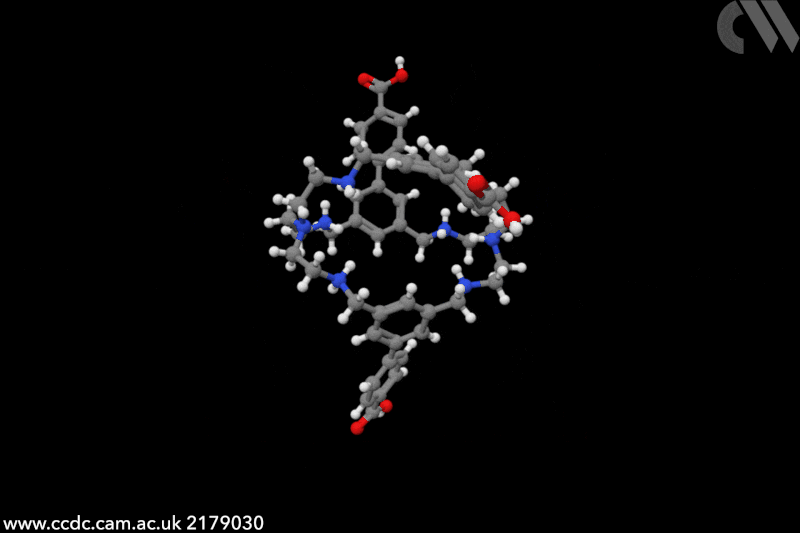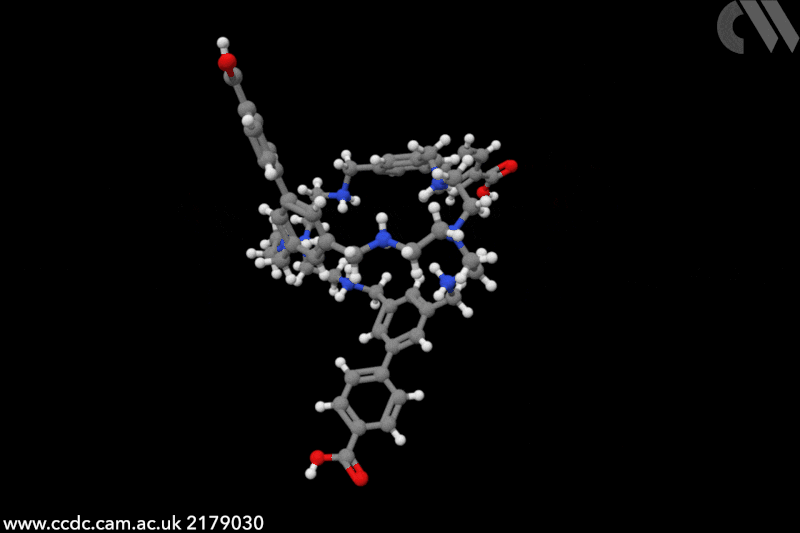
Touchless technology could transform how we interact with computers and other machines. Since the start of the Covid pandemic, interest in touchless sensors has rocketed – market projections predict sales will triple by 2026, to an estimated to an estimated $37.6 billion (£30 billion). Traditionally, touchless sensors use ‘physical’ stimuli such as infrared radiation, ultrasounds or capacitance. In this case, chemistry is key. ‘Humidity sensors detect the presence of a finger, it’s very creative,’ says Jonathan Nitschke, an expert in supramolecular cages at the University of Cambridge, UK. ‘I had never heard of this application before.’
The chemical cages created by Khashab and collaborators feature a handful of functional groups that crave water. In particular, protonated amines cover the cavity’s interior, while the external part is decorated with carboxylic groups – both moisture-sensitive moieties. ‘Human skin naturally releases moisture and [our] sensors capture its variations,’ explains Khashab. Instead of relying on purely proximity or gestures, detecting humidity ‘allows for a more natural and intuitive form of touchless interactions’, she adds. ‘We evaluated the performance of our touchless screens through nearly a hundred trials [and] consistently achieved a 100% rate in correct detection.’
‘This paper is a great engineering demonstration of a chemical discovery,’ says Shushei Furukawa, an expert in supramolecular chemistry at Kyoto University in Japan. In low humidity, the cage surface hosts a small amount of water molecules, constrained by the hydrogen bonds formed with the moisture-sensitive groups. This limits their mobility, which is linked to a high resistance. The increase in humidity makes more water molecules accumulate in the cage, which in turn transforms the network of hydrogen bonds. The phenomenon of ‘water adsorption in porous organic cages is translated into proton conduction and, therefore, a change in resistivity’, he explains.
For Furukawa, this discovery opens the possibility of touchless technology to other materials. ‘Any porous materials that uptake water with high adsorption and desorption kinetics could work,’ he says. This includes metal–organic frameworks (MOFs) and covalent–organic frameworks (COFs), which haven’t yet been explored for such applications. The problem is likely to be processability, explains Furukawa. ‘Thanks to the nature of [this crystalline] cage, it was easily processed into an electronic device, [which] showed a highly robust sensing performance,’ he says.
Arnau Carné Sánchez, a postdoctoral researcher working on supramolecular structures at the Institute of Nanoscience and Nanotechnology in Barcelona, Spain, agrees that processability of this cage is a clear advantage over other materials such as MOFs. ‘One of the main drawbacks of conventional porous materials for manufacturing devices is poor processability,’ he explains. ‘The crystalline cages in this study are soluble in water, which allows the use of state-of-the-art “drop casting” techniques.’ This is a simple strategy to form films, which works by dropping a solution of solids into a surface and then evaporating the solvent. Eventually, the cages are ‘structured onto the sensor surface, facilitating the fabrication of a useful device’, adds Carné Sánchez. When ‘researchers analysed the electrical response as a function of the distance between the device and a finger, they found it was very accurate and reproducible’, he says. Additionally, the fast kinetics of the reaction create an ‘ultrafast response’ and raise the recovery abilities of the sensor. According to the authors, the recovery time hovers between one and three seconds, and the sensor exhibits an ‘exceptional stability’ showing successful measurements throughout over 800 cycles.
As the sensors detect humidity, the device presents problems when ambient conditions exceed certain limits. With an average ambient humidity of 60%, the sensor responds well to a fingertip, Khashab says. However, if the ambient humidity is higher than that near to the skin, the sensor doesn’t work reliably, which would limit its use. ‘The change in resistance is lower, [therefore] sensitivity is lower too, explains Furukawa. However, it’s a first proof-of-concept. In the future, modifications of the supramolecular structure could surpass the disadvantages – maybe creating crystalline cages with different chemical groups that work better in highly humid conditions. Moreover, materials like MOFs and COFs could provide other options too. ‘Different processable porous materials with more hydrophobic pores [could still] adsorb water at higher humidity,’ adds Furukawa.
Khashab’s team has created a simple touchless screen, as well as a touchless ‘password manager’ device, similar to the patterned screen locks on smartphones. The latter incorporates an array of 25 humidity sensors, all responsive to the proximity of a finger – which could increase not only safety, but also security, as it removes the risk of marking the surface with fingerprints. The design of the supramolecular cages ‘suggest the sensor is easily scalable’, says Khashab.
‘They’re relatively cheap materials and fabrication processes,’ explains Nitschke. ‘It looks scalable to me!’
References
J Wang et al, Nat. Commun., 2024, 15, 1575 (DOI: 10.1038/s41467-024-46071-8)














No comments yet