Abstract
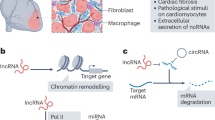
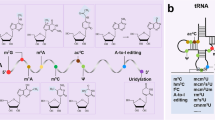
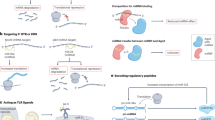
Cardiovascular diseases are the leading cause of death globally and are associated with increasing financial expenditure. With the availability of next-generation sequencing technologies since the early 2000s, non-coding RNAs such as microRNAs, long non-coding RNAs and circular RNAs have been assessed as potential therapeutic targets for numerous diseases, including cardiovascular diseases. In this Review, we summarize current approaches employed to screen for novel coding and non-coding RNA candidates with diagnostic and therapeutic potential in cardiovascular disease, including next-generation sequencing, functional high-throughput RNA screening and single-cell sequencing technologies. Furthermore, we highlight viral-based delivery tools that have been widely used to evaluate the therapeutic utility of both coding and non-coding RNAs in the context of cardiovascular disease. Finally, we discuss the potential of using oligonucleotide-based molecular products such as modified RNA, small interfering RNA and RNA mimics/inhibitors for the treatment of cardiovascular diseases. Given that many non-coding RNAs have not yet been functionally annotated, the number of potential RNA diagnostic and therapeutic targets for cardiovascular diseases will continue to expand for years to come.
Key points
-
RNA sequencing technology has been used to identify non-coding RNAs that might have a role in the pathogenesis of cardiovascular disease and that might be used as treatment targets.
-
Advantages of non-coding RNAs as diagnostic biomarkers include ease of detection in body fluids, cell type-specific expression patterns and fluctuations in expression levels in response to stress or disease.
-
Transcripts of non-coding RNAs can be packaged into viral vectors and delivered into target cells to mediate their therapeutic effect.
-
Synthetic oligonucleotides, such as microRNA mimics and modified mRNAs, have also been gaining more attention as potential RNA delivery tools, given their advantages such as ease of dosage control and low immunogenicity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Benjamin, E. J. et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 135, e146–e603 (2017).
Savarese, G. & Lund, L. H. Global public health burden of heart failure. Card. Fail. Rev. 3, 7–11 (2017).
Sullenger, B. A. & Nair, S. From the RNA world to the clinic. Science 352, 1417–1420 (2016).
Drusco, A. & Croce, C. M. MicroRNAs and cancer: a long story for short RNAs. Adv. Cancer Res. 135, 1–24 (2017).
Thum, T. Noncoding RNAs and myocardial fibrosis. Nat. Rev. Cardiol. 11, 655–663 (2014).
Devaux, Y. et al. Long noncoding RNAs in cardiac development and ageing. Nat. Rev. Cardiol. 12, 415–425 (2015).
Zheng, G. X. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Kolodziejczyk, A. A., Kim, J. K., Svensson, V., Marioni, J. C. & Teichmann, S. A. The technology and biology of single-cell RNA sequencing. Mol. Cell 58, 610–620 (2015).
Han, Y., Gao, S., Muegge, K., Zhang, W. & Zhou, B. Advanced applications of RNA sequencing and challenges. Bioinform. Biol. Insights 9, 29–46 (2015).
Robertson, G. et al. De novo assembly and analysis of RNA-seq data. Nat. Methods 7, 909–912 (2010).
Tang, F. et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009).
Frumkin, D. et al. Amplification of multiple genomic loci from single cells isolated by laser micro-dissection of tissues. BMC Biotechnol. 8, 17 (2008).
Hayashi, T. et al. Single-cell gene profiling of planarian stem cells using fluorescent activated cell sorting and its “index sorting” function for stem cell research. Dev. Growth Differ. 52, 131–144 (2010).
Hashimshony, T., Wagner, F., Sher, N. & Yanai, I. CEL-Seq: single-cell RNA-Seq by multiplexed linear amplification. Cell Rep. 2, 666–673 (2012).
Picelli, S. et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods 10, 1096–1098 (2013).
Lee, R. C., Feinbaum, R. L. & Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854 (1993).
Pasquinelli, A. E. et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 408, 86–89 (2000).
Rao, P. K. et al. Loss of cardiac microRNA-mediated regulation leads to dilated cardiomyopathy and heart failure. Circ. Res. 105, 585–594 (2009).
Sayed, D., Hong, C., Chen, I. Y., Lypowy, J. & Abdellatif, M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ. Res. 100, 416–424 (2007).
Liu, X. et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 21, 584–595 (2015).
Akat, K. M. et al. Comparative RNA-sequencing analysis of myocardial and circulating small RNAs in human heart failure and their utility as biomarkers. Proc. Natl Acad. Sci. USA 111, 11151–11156 (2014).
Lopez, J. P. et al. Biomarker discovery: quantification of microRNAs and other small non-coding RNAs using next generation sequencing. BMC Med. Genomics 8, 35 (2015).
Freedman, J. E. et al. Diverse human extracellular RNAs are widely detected in human plasma. Nat. Commun. 7, 11106 (2016).
Danielson, K. M. et al. Plasma circulating extracellular RNAs in left ventricular remodeling post-myocardial infarction. EBioMedicine 32, 172–181 (2018).
Neumann, A. et al. MicroRNA 628-5p as a novel biomarker for cardiac allograft vasculopathy. Transplantation 101, e26–e33 (2017).
Wang, G. K. et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur. Heart J. 31, 659–666 (2010).
Bar, C., Chatterjee, S. & Thum, T. Long noncoding RNAs in cardiovascular pathology, diagnosis, and therapy. Circulation 134, 1484–1499 (2016).
Lee, J. H. et al. Analysis of transcriptome complexity through RNA sequencing in normal and failing murine hearts. Circ. Res. 109, 1332–1341 (2011).
Yang, K. C. et al. Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation 129, 1009–1021 (2014).
Ounzain, S. et al. Genome-wide profiling of the cardiac transcriptome after myocardial infarction identifies novel heart-specific long non-coding RNAs. Eur. Heart J. 36, 353–368a (2015).
Kaikkonen, M. U. et al. Genome-wide dynamics of nascent noncoding RNA transcription in porcine heart after myocardial infarction. Circ. Cardiovasc. Genet. 10, e001702 (2017).
Fiedler, J. et al. Development of long noncoding RNA-based strategies to modulate tissue vascularization. J. Am. Coll. Cardiol. 66, 2005–2015 (2015).
Kumarswamy, R. et al. Circulating long noncoding RNA, LIPCAR, predicts survival in patients with heart failure. Circ. Res. 114, 1569–1575 (2014).
de Gonzalo-Calvo, D. et al. Circulating long-non coding RNAs as biomarkers of left ventricular diastolic function and remodelling in patients with well-controlled type 2 diabetes. Sci. Rep. 6, 37354 (2016).
Gu, M. et al. Circulating LncRNAs as novel, non-invasive biomarkers for prenatal detection of fetal congenital heart defects. Cell. Physiol. Biochem. 38, 1459–1471 (2016).
Kitow, J. et al. Mitochondrial long noncoding RNAs as blood based biomarkers for cardiac remodeling in patients with hypertrophic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 311, H707–H712 (2016).
Xu, Y. J., Huang, R. T., Gu, J. N. & Jiang, W. F. Identification of long non-coding RNAs as novel biomarker and potential therapeutic target for atrial fibrillation in old adults. Oncotarget 7, 10803–10811 (2016).
Yan, Y. Y. et al. Circulating long noncoding RNA UCA1 as a novel biomarker of acute myocardial infarction. Biomed. Res. Int. 2016, 8079372 (2016).
Gao, L. et al. Circulating long noncoding RNA HOTAIR is an essential mediator of acute myocardial infarction. Cell Physiol. Biochem. 44, 1497–1508 (2017).
Xuan, L. N. et al. Circulating long non-coding RNAs NRON and MHRT as novel predictive biomarkers of heart failure. J. Cell. Mol. Med. 21, 1803–1814 (2017).
Gupta, S. K. et al. Quaking inhibits doxorubicin-mediated cardiotoxicity through regulation of cardiac circular RNA expression. Circ. Res. 122, 246–254 (2018).
Aufiero, S., Reckman, Y. J., Pinto, Y. M. & Creemers, E. E. Circular RNAs open a new chapter in cardiovascular biology. Nat. Rev. Cardiol. https://doi.org/10.1038/s41569-019-0185-2 (2019).
Nigro, J. M. et al. Scrambled exons. Cell 64, 607–613 (1991).
Sanger, H. L., Klotz, G., Riesner, D., Gross, H. J. & Kleinschmidt, A. K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl Acad. Sci. USA 73, 3852–3856 (1976).
Kos, A., Dijkema, R., Arnberg, A. C., van der Meide, P. H. & Schellekens, H. The hepatitis delta (delta) virus possesses a circular RNA. Nature 323, 558–560 (1986).
Jeck, W. R. & Sharpless, N. E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 32, 453–461 (2014).
Jia, W., Xu, B. & Wu, J. Circular RNA expression profiles of mouse ovaries during postnatal development and the function of circular RNA epidermal growth factor receptor in granulosa cells. Metabolism 85, 192–204 (2018).
Legnini, I. et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol. Cell 66, 22–37 (2017).
Li, L., Guo, J., Chen, Y., Chang, C. & Xu, C. Comprehensive CircRNA expression profile and selection of key CircRNAs during priming phase of rat liver regeneration. BMC Genomics 18, 80 (2017).
Luo, J. et al. Profiling circRNA and miRNA of radiation-induced esophageal injury in a rat model. Sci. Rep. 8, 14605 (2018).
Zeng, Y. et al. Altered expression profiles of circular RNA in colorectal cancer tissues from patients with lung metastasis. Int. J. Mol. Med. 40, 1818–1828 (2017).
Cao, Y. et al. Changing expression profiles of long non-coding RNAs, mRNAs and circular RNAs in ethylene glycol-induced kidney calculi rats. BMC Genomics 19, 660 (2018).
Werfel, S. et al. Characterization of circular RNAs in human, mouse and rat hearts. J. Mol. Cell Cardiol. 98, 103–107 (2016).
Tan, W. L. et al. A landscape of circular RNA expression in the human heart. Cardiovasc. Res. 113, 298–309 (2017).
Wu, H. J., Zhang, C. Y., Zhang, S., Chang, M. & Wang, H. Y. Microarray expression profile of circular RNAs in heart tissue of mice with myocardial infarction-induced heart failure. Cell. Physiol. Biochem. 39, 205–216 (2016).
Tang, C. M. et al. CircRNA_000203 enhances the expression of fibrosis-associated genes by derepressing targets of miR-26b-5p, Col1a2 and CTGF, in cardiac fibroblasts. Sci. Rep. 7, 40342 (2017).
Zhou, B. & Yu, J. W. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-beta1. Biochem. Biophys. Res. Commun. 487, 769–775 (2017).
Siede, D. et al. Identification of circular RNAs with host gene-independent expression in human model systems for cardiac differentiation and disease. J. Mol. Cell Cardiol. 109, 48–56 (2017).
Shang, F. F., Luo, S., Liang, X. & Xia, Y. Alterations of circular RNAs in hyperglycemic human endothelial cells. Biochem. Biophys. Res. Commun. 499, 551–555 (2018).
Alhasan, A. A. et al. Circular RNA enrichment in platelets is a signature of transcriptome degradation. Blood 127, e1–e11 (2016).
Maass, P. G. et al. A map of human circular RNAs in clinically relevant tissues. J. Mol. Med. 95, 1179–1189 (2017).
Lei, W. et al. Signature of circular RNAs in human induced pluripotent stem cells and derived cardiomyocytes. Stem Cell Res. Ther. 9, 56 (2018).
Devaux, Y. et al. Circular RNAs in heart failure. Eur. J. Heart Fail. 19, 701–709 (2017).
Vausort, M. et al. Myocardial infarction-associated circular RNA predicting left ventricular dysfunction. J. Am. Coll. Cardiol. 68, 1247–1248 (2016).
Salgado-Somoza, A., Zhang, L., Vausort, M. & Devaux, Y. The circular RNA MICRA for risk stratification after myocardial infarction. Int. J. Cardiol. Heart Vasc. 17, 33–36 (2017).
Liu, Z. Q. et al. Single-cell transcriptomics reconstructs fate conversion from fibroblast to cardiomyocyte. Nature 551, 100–104 (2017).
Lescroart, F. et al. Defining the earliest step of cardiovascular lineage segregation by single-cell RNA-seq. Science 359, 1177–1181 (2018).
Gladka, M. M. et al. Single-cell sequencing of the healthy and diseased heart reveals Ckap4 as a new modulator of fibroblasts activation. Circulation 138, 166–180 (2018).
Blakely, K., Ketela, T. & Moffat, J. Pooled lentiviral shRNA screening for functional genomics in mammalian cells. Methods Mol. Biol. 781, 161–182 (2011).
Zhu, S. et al. Genome-scale deletion screening of human long non-coding RNAs using a paired-guide RNA CRISPR-Cas9 library. Nat. Biotechnol. 34, 1279–1286 (2016).
Fei, T. et al. Genome-wide CRISPR screen identifies HNRNPL as a prostate cancer dependency regulating RNA splicing. Proc. Natl Acad. Sci. USA 114, E5207–E5215 (2017).
Haney, S. A. High-content screening approaches that minimize confounding factors in RNAi, CRISPR, and small molecule screening. Methods Mol. Biol. 1683, 113–130 (2018).
Eulalio, A. et al. Functional screening identifies mi-RNAs inducing cardiac regeneration. Nature 492, 376–381 (2012).
Ucar, A. et al. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nat. Commun. 3, 1078 (2012).
Fiedler, J. et al. Functional MicroRNA library screening identifies the HypoxaMiR MiR-24 as a potent regulator of smooth muscle cell proliferation and vascularization. Antioxid. Redox Signal. 21, 1167–1176 (2014).
Gupta, S. K. et al. Preclinical development of a microRNA-based therapy for elderly patients with myocardial infarction. J. Am. Coll. Cardiol. 68, 1557–1571 (2016).
Krausz, E. High-content siRNA screening. Mol. Biosyst 3, 232–240 (2007).
Jiang, X. et al. A novel EST-derived RNAi screen reveals a critical role for farnesyl diphosphate synthase in beta2-adrenergic receptor internalization and down-regulation. FASEB J. 26, 1995–2007 (2012).
Willingham, A. T. et al. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science 309, 1570–1573 (2005).
Beermann, J. et al. A large shRNA library approach identifies lncRNA Ntep as an essential regulator of cell proliferation. Cell Death Differ. 25, 307–318 (2018).
Pfeifer, A. & Verma, I. M. Gene therapy: promises and problems. Annu. Rev. Genom. Hum. Genet. 2, 177–211 (2001).
French, B. A., Mazur, W., Geske, R. S. & Bolli, R. Feasibility and limitations of direct in-vivo gene-transfer into porcine myocardium using replication-deficient adenoviral vectors. Circulation 90, 517–517 (1994).
Li, X. et al. Loss of AZIN2 splice variant facilitates endogenous cardiac regeneration. Cardiovasc. Res. 114, 1642–1655 (2018).
Wang, K. et al. The long noncoding RNA CHRF regulates cardiac hypertrophy by targeting miR-489. Circ. Res. 114, 1377–1388 (2014).
Li, X. R., Zhou, J. & Huang, K. Inhibition of the lncRNA Mirt1 attenuates acute myocardial infarction by suppressing NF-kappa B activation. Cell. Physiol. Biochem. 42, 1153–1164 (2017).
Wang, K. et al. Circular RNA mediates cardiomyocyte death via miRNA-dependent upregulation of MTP18 expression. Cell Death Differ. 24, 1111–1120 (2017).
Wang, K. et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur. Heart J. 37, 2602–2611 (2016).
Wang, X. H. et al. Increased expression of microRNA-146a decreases myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 97, 432–442 (2013).
Li, Q. L. et al. Overexpression of microRNA-99a attenuates heart remodelling and improves cardiac performance after myocardial infarction. J. Cell. Mol. Med. 18, 919–928 (2014).
Nathwani, A. C. et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 365, 2357–2365 (2011).
Tao, L. et al. Crucial role of miR-433 in regulating cardiac fibrosis. Theranostics 6, 2068–2083 (2016).
Long, Q. Q. et al. Long noncoding RNA Kcna2 antisense RNA contributes to ventricular arrhythmias via silencing Kcna2 in rats with congestive heart failure. J. Am. Heart Assoc. 6, e005965 (2017).
Zhang, J. et al. Long noncoding RNA upregulated in hypothermia treated cardiomyocytes protects against myocardial infarction through improving mitochondrial function. Int. J. Cardiol. 266, 213–217 (2018).
Bar, C. et al. Telomerase expression confers cardioprotection in the adult mouse heart after acute myocardial infarction. Nat. Commun. 5, 5863 (2014).
Ganesan, J. et al. MiR-378 controls cardiac hypertrophy by combined repression of mitogen-activated protein kinase pathway factors. Circulation 127, 2097–2106 (2013).
Quattrocelli, M. et al. Long-term miR-669a therapy alleviates chronic dilated cardiomyopathy in dystrophic mice. J. Am. Heart Assoc. 2, e000284 (2013).
Wu, Z., Yang, H. & Colosi, P. Effect of genome size on AAV vector packaging. Mol. Ther. 18, 80–86 (2010).
Ferreira, V. et al. Immune responses to intramuscular administration of alipogene tiparvovec (AAV1-LPL(S447X)) in a phase II clinical trial of lipoprotein lipase deficiency gene therapy. Hum. Gene Ther. 25, 180–188 (2014).
Calcedo, R., Vandenberghe, L. H., Gao, G., Lin, J. & Wilson, J. M. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 199, 381–390 (2009).
Manno, C. S. et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 12, 342–347 (2006).
Kormann, M. S. et al. Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat. Biotechnol. 29, 154–157 (2011).
Beermann, J., Piccoli, M. T., Viereck, J. & Thum, T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol. Rev. 96, 1297–1325 (2016).
Sokilde, R., Newie, I., Persson, H., Borg, A. & Rovira, C. Passenger strand loading in overexpression experiments using microRNA mimics. RNA Biol. 12, 787–791 (2015).
Pankratz, F. et al. MicroRNA-100 suppresses chronic vascular inflammation by stimulation of endothelial autophagy. Circ. Res. 122, 417–432 (2018).
Lesizza, P. et al. Single-dose intracardiac injection of pro-regenerative microRNAs improves cardiac function after myocardial infarction. Circ. Res. 120, 1298–1304 (2017).
Yuan, J. et al. MicroRNA-378 suppresses myocardial fibrosis through a paracrine mechanism at the early stage of cardiac hypertrophy following mechanical stress. Theranostics 8, 2565–2582 (2018).
Shi, J. et al. miR-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia reperfusion injury. Theranostics 7, 664–676 (2017).
Bader, A. G., Brown, D., Stoudemire, J. & Lammers, P. Developing therapeutic microRNAs for cancer. Gene Ther. 18, 1121–1126 (2011).
Takeshita, F. et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol. Ther. 18, 181–187 (2010).
Sanganalmath, S. K. & Bolli, R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ. Res. 113, 810–834 (2013).
Chien, K. R., Zangi, L. & Lui, K. O. Synthetic chemically modified mRNA (modRNA): toward a new technology platform for cardiovascular biology and medicine. Cold Spring Harb. Perspect. Med. 5, a014035 (2014).
Zangi, L. et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 31, 898–907 (2013).
Huang, C. L. et al. Synthetic chemically modified mrna-based delivery of cytoprotective factor promotes early cardiomyocyte survival post-acute myocardial infarction. Mol. Pharm. 12, 991–996 (2015).
Rinaldi, C. & Wood, M. J. A. Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 14, 9–21 (2018).
Evers, M. M., Toonen, L. J. & van Roon-Mom, W. M. Antisense oligonucleotides in therapy for neurodegenerative disorders. Adv. Drug Deliv. Rev. 87, 90–103 (2015).
Singh, J., Kaur, H., Kaushik, A. & Peer, S. A. Review of antisense therapeutic interventions for molecular biological targets in various diseases. Int. J. Pharmacol. 7, 294–315 (2011).
Graham, M. J. et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N. Engl. J. Med. 377, 222–232 (2017).
Krutzfeldt, J. et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438, 685–689 (2005).
Czech, M. P. MicroRNAs as therapeutic targets. N. Engl. J. Med. 354, 1194–1195 (2006).
Thum, T. et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456, 980–U983 (2008).
Kaur, H., Arora, A., Wengel, J. & Maiti, S. Thermodynamic, counterion, and hydration effects for the incorporation of locked nucleic acid nucleotides into DNA duplexes. Biochemistry 45, 7347–7355 (2006).
Michalik, K. M. et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ. Res. 114, 1389–1397 (2014).
Chery, J. RNA therapeutics: RNAi and antisense mechanisms and clinical applications. Postdoc J. 4, 35–50 (2016).
Martinez, E. C. et al. MicroRNA-31 promotes adverse cardiac remodeling and dysfunction in ischemic heart disease. J. Mol. Cell. Cardiol. 112, 27–39 (2017).
Zhou, Q. et al. Inhibition of miR-208b improves cardiac function in titin-based dilated cardiomyopathy. Int. J. Cardiol. 230, 634–641 (2017).
Di Gregoli, K. et al. MicroRNA-181b controls atherosclerosis and aneurysms through regulation of TIMP-3 and elastin. Circ. Res. 120, 49–65 (2017).
DeVos, S. L. & Miller, T. M. Antisense oligonucleotides: treating neurodegeneration at the level of RNA. Neurotherapeutics 10, 486–497 (2013).
Viereck, J. et al. Long noncoding RNA Chast promotes cardiac remodeling. Sci. Transl Med. 8, 326ra22 (2016).
Piccoli, M. T. et al. Inhibition of the cardiac fibroblast-enriched lncRNA Meg3 prevents cardiac fibrosis and diastolic dysfunction. Circ. Res. 121, 575–583 (2017).
Poller, W., Tank, J., Skurk, C. & Gast, M. Cardiovascular RNA interference therapy: the broadening tool and target spectrum. Circ. Res. 113, 588–602 (2013).
Wang, Z. et al. The long noncoding RNA Chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat. Med. 22, 1131–1139 (2016).
Wu, H., Liu, J., Li, W., Liu, G. & Li, Z. LncRNA-HOTAIR promotes TNF-alpha production in cardiomyocytes of LPS-induced sepsis mice by activating NF-kappaB pathway. Biochem. Biophys. Res. Commun. 471, 240–246 (2016).
Du, W. W. et al. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur. Heart J. 38, 1402–1412 (2017).
Watts, J. K. & Corey, D. R. Silencing disease genes in the laboratory and the clinic. J. Pathol. 226, 365–379 (2012).
Willms, E. et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 6, 22519 (2016).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Ha, D., Yang, N. & Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm. Sin. B 6, 287–296 (2016).
Tran, T. H., Mattheolabakis, G., Aldawsari, H. & Amiji, M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin. Immunol. 160, 46–58 (2015).
Goldie, B. J. et al. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 42, 9195–9208 (2014).
Sahoo, S. et al. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ. Res. 109, 724–728 (2011).
Yu, B. et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int. J. Cardiol. 182, 349–360 (2015).
Bang, C. et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J. Clin. Invest. 124, 2136–2146 (2014).
Ong, S. G. et al. Cross talk of combined gene and cell therapy in ischemic heart disease role of exosomal microRNA transfer. Circulation 130, S60–S69 (2014).
Willis, G. R., Kourembanas, S. & Mitsialis, S. A. Toward exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front. Cardiovasc. Med. 4, 63 (2017).
Danhier, F. et al. PLGA-based nanoparticles: an overview of biomedical applications. J. Control. Release 161, 505–522 (2012).
Farokhzad, O. C. & Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 3, 16–20 (2009).
Gadde, S. & Rayner, K. J. Nanomedicine meets microRNA: current advances in RNA-based nanotherapies for atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 36, e73–e79 (2016).
Duivenvoorden, R. et al. Nanoimmunotherapy to treat ischaemic heart disease. Nat. Rev. Cardiol. 16, 21–32 (2019).
Hartmann, D. et al. MicroRNA-based therapy of GATA2-deficient vascular disease. Circulation 134, 1973–1990 (2016).
Deng, S. et al. Neonatal heart-enriched miR-708 promotes proliferation and stress resistance of cardiomyocytes in rodents. Theranostics 7, 1953–1965 (2017).
Jia, C. et al. Gold nanoparticle-based miR155 antagonist macrophage delivery restores the cardiac function in ovariectomized diabetic mouse model. Int. J. Nanomed. 12, 4963–4979 (2017).
Zeng, Y. et al. A circular RNA binds to and activates AKT phosphorylation and nuclear localization reducing apoptosis and enhancing cardiac repair. Theranostics 7, 3842–3855 (2017).
Leuschner, F. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat. Biotechnol. 29, 1005–1010 (2011).
Acknowledgements
T.T. is supported by grants from the European Research Council (consolidator grant), the European Union (EU) Horizon 2020 programme (for CardioReGenix), the ERANet CVD (project EXPERT) and the Deutsche Forschungsgemeinschaft (Th903/19-1, Th903/20-1 and Th903/22-1). The authors thank C. Bär and S. Chatterjee (Institute of Molecular and Translational Therapeutic Strategies, Hannover Medical School, Hannover, Germany) for help with manuscript editing.
Reviewer information
Nature Reviews Cardiology thanks K. Rayner, F. Martellli and the other anonymous reviewer(s), for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
Both authors researched the data for this article, discussed the content, wrote the manuscript and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
T.T. has filed several microRNA-based and long-non-coding-RNA-based patents in cardiovascular medicine and is the founder and shareholder of Cardior Pharmaceuticals GmbH. D.L. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Non-coding RNAs
-
RNA molecules that are not translated into a protein.
- microRNAs
-
(miRNAs). Endogenous, small non-coding RNAs ~20–22 nucleotides in length that can regulate gene expression.
- Long non-coding RNAs
-
(lncRNAs). Non-coding RNA transcripts >200 nucleotides in length.
- Circular RNAs
-
(circRNAs). Single-stranded, non-coding RNA transcripts that form a covalently closed continuous loop.
- mRNA
-
A large family of RNA molecules that transfer genetic information from DNA to the ribosome.
- Short hairpin RNA
-
(shRNA). An artificially engineered RNA molecule with a tight hairpin turn that can be used to silence gene expression.
- miRNA mimics
-
Chemically modified RNAs that mimic endogenous microRNAs.
- miRNA sponge
-
RNA transcripts containing multiple high-affinity microRNA (miRNA)-binding sequences that can sequester miRNAs from their endogenous target mRNAs.
- Small interfering RNA
-
(siRNA). Small RNA transcripts ~20–22 nucleotides in length that can be used to disrupt the translation of proteins by binding to and promoting the degradation of mRNA at specific sequences.
- Modified mRNA
-
(modRNA). Chemically modified mRNA with improved protein translation capacity, which is accomplished by reducing potential mutagenic and immunological effects.
- Antisense oligonucleotides
-
(ASOs). Short, synthetic oligonucleotides ~15–25 nucleotides in length designed to bind to and degrade complementary RNA targets.
- Locked nucleic acid
-
(LNA). A synthetic nucleic acid analogue containing a bridged, bicyclic sugar moiety, which has high affinity and specificity for complementary RNA and DNA.
Rights and permissions
About this article
Cite this article
Lu, D., Thum, T. RNA-based diagnostic and therapeutic strategies for cardiovascular disease. Nat Rev Cardiol 16, 661–674 (2019). https://doi.org/10.1038/s41569-019-0218-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-019-0218-x
This article is cited by
-
DNA-based molecular classifiers for the profiling of gene expression signatures
Journal of Nanobiotechnology (2024)
-
Non-coding RNAs in disease: from mechanisms to therapeutics
Nature Reviews Genetics (2024)
-
RNA-binding proteins in cardiovascular biology and disease: the beat goes on
Nature Reviews Cardiology (2024)
-
Differential expression profiles and functional analysis of long non-coding RNAs in calcific aortic valve disease
BMC Cardiovascular Disorders (2023)
-
Insulin-like growth factor binding protein 2: a core biomarker of left ventricular dysfunction in dilated cardiomyopathy
Hereditas (2023)