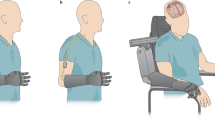
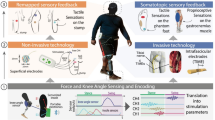
Abstract
Commercial prosthetic devices currently do not provide natural sensory information on the interaction with objects or movements. The subsequent disadvantages include unphysiological walking with a prosthetic leg and difficulty in controlling the force exerted with a prosthetic hand, thus creating health issues. Restoring natural sensory feedback from the prosthesis to amputees is an unmet clinical need. An optimal device should be able to elicit natural sensations of touch or proprioception, by delivering the complex signals to the nervous system that would be produced by skin, muscles and joints receptors. This Review covers the various neurotechnological approaches that have been proposed for the development of the optimal sensory feedback restoration device for arm and leg amputees.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


a, Bottom left (grey hand) and far right (brain), reproduced with permission from pixy.org; bottom right (remapping approach), adapted with permission from iStock by Getty Images/Francesco Maria Petrini; bottom, second from left (sensorized glove), reproduced with permission from ref. 75, under a Creative Commons license CC BY 4.0; top, second from left (sensorized insole), adapted with permission from ref. 84, AAAS. b, Left, reproduced with permission from ref. 109, Elsevier. d, Hands, adapted with permission from ref. 112, Elsevier Biomedical Press; feet, adapted with permission from ref. 18, American Physiological Society

panels reproduced with permission from: a,j, ref. 113, under a Creative Commons license CC BY 4.0; b,f, ref. 39, IOP; c, ref. 114, IEEE; d, ref. 40, Elsevier; e, ref. 49, Wiley; g,h, ref. 54, IEEE; i, ref. 63, Springer Nature Ltd; l,m, ref. 116, River Publishers; n, ref. 56, IEEE. Panel k adapted with permission from ref. 115, IOP

TSR sensation location, reproduced with permission from ref. 80, under a Creative Commons license CC BY 4.0; cuff sensation location and plot, adapted with permission from ref. 69, AAAS; FINE sensation location and plot, adapted with permission from ref. 53, AAAS; tf-LIFE sensation location and picture, adapted with permission from ref. 41, IOP; TIME sensation location and plot, reproduced with permission from ref. 10, Wiley; USEA sensation location, reproduced with permission from ref. 71, IOP; USEA plot, adapted with permission from ref. 66, AAAS; TSR, FINE and USEA pictures, adapted with permission from ref. 99, under a Creative Commons license CC BY-NC-ND 4.0

TIME sensation location, reproduced with permission from ref. 84, AAAS; FINE picture, adapted with permission from ref. 99, under a Creative Commons license CC BY-NC-ND 4.0; AMI sensation location and plot, adapted with permission from ref. 86, AAAS; FINE sensation location, adapted with permission from ref. 82, under a Creative Commons license CC BY 3.0
Similar content being viewed by others
Data availability
The data and calculations that support the findings of this Review are available from the corresponding authors upon reasonable request.
References
Unwin, N. Epidemiology of lower extremity amputation in centres in Europe, North America and East Asia. Br. J. Surg. 87, 328–337 (2000).
Moxey, P. W. et al. Lower extremity amputations—a review of global variability in incidence. Diabet. Med. 28, 1144–1153 (2011).
Winkler, S. L. H. in Care of the Combat Amputee (eds Pasquina, P. F. et al.) 597–605 (Department of the Army, 2009).
Sanità solo il 5 di amputazioni è legata a infortuni sul lavoro. INAIL https://www.inail.it/cs/internet/comunicazione/news-ed-eventi/news/p1780018061_sanita_solo_il_5_di_amputazi.html (2010).
Atroshi, I. & Rosberg, H. E. Epidemiology of amputations and severe injuries of the hand. Hand Clin. 17, 343–350 (2001).
Miller, W. C., Speechley, M. & Deathe, B. The prevalence and risk factors of falling and fear of falling among lower extremity amputees. Arch. Phys. Med. Rehabil. 82, 1031–1037 (2001).
Nolan, L. et al. Adjustments in gait symmetry with walking speed in trans-femoral and trans-tibial amputees. Gait Posture 17, 142–151 (2003).
Waters, R. L., Perry, J., Antonelli, D. & Hislop, H. Energy cost of walking of amputees: the influence of level of amputation. J. Bone Joint Surg. Am. 58, 42–46 (1976).
Biddiss, E., Beaton, D. & Chau, T. Consumer design priorities for upper limb prosthetics. Disabil. Rehabil. Assist. Technol. 2, 346–357 (2007).
Petrini, F. M. et al. Six-month assessment of a hand prosthesis with intraneural tactile feedback. Ann. Neurol. 85, 137–154 (2019).
Williamson, G. M., Schulz, R., Bridges, M. W. & Behan, A. M. Social and psychological factors in adjustment to limb amputation. J. Soc. Behav. Pers. 9, 249–268 (1994).
Blanke, O. Multisensory brain mechanisms of bodily self-consciousness. Nat. Rev. Neurosci. 13, 556–571 (2012).
Heller, B. W., Datta, D. & Howitt, J. A pilot study comparing the cognitive demand of walking for transfemoral amputees using the intelligent prosthesis with that using conventionally damped knees. Clin. Rehabil. 14, 518–522 (2000).
Makin, T. R., de Vignemont, F. & Faisal, A. A. Neurocognitive barriers to the embodiment of technology. Nat. Biomed. Eng. 1, 0014 (2017).
Flor, H., Nikolajsen, L. & Staehelin Jensen, T. Phantom limb pain: a case of maladaptive CNS plasticity? Nat. Rev. Neurosci. 7, 873–881 (2006).
Roffman, C. E., Buchanan, J. & Allison, G. T. Predictors of non-use of prostheses by people with lower limb amputation after discharge from rehabilitation: development and validation of clinical prediction rules. J. Physiother. 60, 224–231 (2014).
Torebjörk, H. E., Vallbo, A. A. B. & Ochoa, J. L. Intraneural microstimulation in man: its relation to specificity of tactile sensations. Brain 110, 1509–1529 (1987).
Strzalkowski, N. D., Peters, R. M., Inglis, J. T. & Bent, L. R. Cutaneous afferent innervation of the human foot sole: what can we learn from single-unit recordings? J. Neurophysiol. 120, 1233–1246 (2018).
Muniak, M. A., Ray, S., Hsiao, S. S., Dammann, J. F. & Bensmaia, S. J. The neural coding of stimulus intensity: linking the population response of mechanoreceptive afferents with psychophysical behavior. J. Neurosci. 27, 11687–11699 (2007).
Weber, A. I. et al. Spatial and temporal codes mediate the tactile perception of natural textures. Proc. Natl Acad. Sci. USA 110, 17107–17112 (2013).
Johansson, R. S. & Birznieks, I. First spikes in ensembles of human tactile afferents code complex spatial fingertip events. Nat. Neurosci. 7, 170–177 (2004).
Proske, U. & Gandevia, S. C. The proprioceptive senses: their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 92, 1651–1697 (2012).
Macefield, G., Gandevia, S. C. & Burke, D. Perceptual responses to microstimulation of single afferents innervating joints, muscles and skin of the human hand. J. Physiol. 429, 113–129 (1990).
Rusaw, D., Hagberg, K., Nolan, L. & Ramstrand, N. Can vibratory feedback be used to improve postural stability in persons with transtibial limb loss? J. Rehabil. Res. Dev. 49, 1239–1254 (2012).
Dietrich, C. et al. Leg prosthesis with somatosensory feedback reduces phantom limb pain and increases functionality. Front. Neurol. 9, 270 (2018).
Shannon, G. F. A myoelectrically-controlled prosthesis with sensory feedback. Med. Biol. Eng. Comput. 17, 73–80 (1979).
Prior, R. E. Supplemental sensory feedback for the VA/NU myoelectric hand background and preliminary designs. Bull. Prosthet. Res. 22, 170–91 (1976).
Dosen, S., Schaeffer, M.-C. & Farina, D. Time-division multiplexing for myoelectric closed-loop control using electrotactile feedback. J. Neuroeng. Rehabil. 11, 138 (2014).
Naples, G. G., Mortimer, J. T., Scheiner, A. & Sweeney, J. D. A spiral nerve cuff electrode for peripheral nerve stimulation. IEEE Trans. Biomed. Eng. 35, 905–916 (1988).
Tyler, D. J. & Durand, D. M. Functionally selective peripheral nerve stimulation with a flat interface nerve electrode. IEEE Trans. Neural Syst. Rehabil. Eng. 10, 294–303 (2002).
McNaughton, T. G. & Horch, K. W. Metallized polymer fibers as leadwires and intrafascicular microelectrodes. J. Neurosci. Methods 70, 103–107 (1996).
Poppendieck, W. et al. A new generation of double-sided intramuscular electrodes for multi-channel recording and stimulation. In 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 7135–7138 (IEEE, 2015).
Wark, H. A. C. et al. A new high-density (25 electrodes/mm2) penetrating microelectrode array for recording and stimulating sub-millimeter neuroanatomical structures. J. Neural Eng. 10, 045003 (2013).
Boretius, T. et al. A transverse intrafascicular multichannel electrode (TIME) to interface with the peripheral nerve. Biosens. Bioelectron. 26, 62–69 (2010).
Hassler, C., Boretius, T. & Stieglitz, T. Polymers for neural implants. J. Polym. Sci. B 49, 18–33 (2011).
Ordonez, J., Schuettler, M., Boehler, C., Boretius, T. & Stieglitz, T. Thin films and microelectrode arrays for neuroprosthetics. MRS Bull. 37, 590–598 (2012).
Ortiz-Catalan, M., Mastinu, E., Sassu, P., Aszmann, O. & Brånemark, R. Self-contained neuromusculoskeletal arm prostheses. N. Engl. J. Med. 382, 1732–1738 (2020).
Badia, J., Pascual-Font, A., Vivó, M., Udina, E. & Navarro, X. Topographical distribution of motor fascicles in the sciatic-tibial nerve of the rat. Muscle Nerve 42, 192–201 (2010).
Freeberg, M. J., Stone, M. A., Triolo, R. J. & Tyler, D. J. The design of and chronic tissue response to a composite nerve electrode with patterned stiffness. J. Neural Eng. 14, 036022 (2017).
Lawrence, S. M., Dhillon, G. S. & Horch, K. W. Fabrication and characteristics of an implantable, polymer-based, intrafascicular electrode. J. Neurosci. Methods 131, 9–26 (2003).
Overstreet, C. K., Cheng, J. & Keefer, E. Fascicle specific targeting for selective peripheral nerve stimulation. J. Neural Eng. https://doi.org/10.1088/1741-2552/ab4370 (2019).
Wurth, S. et al. Long-term usability and bio-integration of polyimide-based intra-neural stimulating electrodes. Biomaterials 122, 114–129 (2017).
Merrill, D. R., Bikson, M. & Jefferys, J. G. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J. Neurosci. Methods 141, 171–198 (2005).
Cogan, S. F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 10, 275–309 (2008).
Rossini, P. M. et al. Double nerve intraneural interface implant on a human amputee for robotic hand control. Clin. Neurophysiol. 121, 777–783 (2010).
Boretius, T. et al. A transverse intrafascicular multichannel electrode (TIME) to treat phantom limb pain—towards human clinical trials. In 2012 4th IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics (BioRob) 282–287 (IEEE, 2012).
Rose, T. L. & Robblee, L. S. Electrical stimulation with Pt electrodes. VIII. Electrochemically safe charge injection limits with 0.2 ms pulses (neuronal application). IEEE Trans. Biomed. Eng. 37, 1118–1120 (1990).
Cogan, S. F. et al. Sputtered iridium oxide films for neural stimulation electrodes. J. Biomed. Mater. Res. B 89, 353–361 (2009).
Grill, W. M. & Mortimer, J. T. Neural and connective tissue response to long-term implantation of multiple contact nerve cuff electrodes. J. Biomed. Mater. Res. 50, 215–226 (2000).
Polasek, K. H., Hoyen, H. A., Keith, M. W., Kirsch, R. F. & Tyler, D. J. Stimulation stability and selectivity of chronically implanted multicontact nerve cuff electrodes in the human upper extremity. IEEE Trans. Neural Syst. Rehabil. Eng. 17, 428–437 (2009).
Tyler, D. J. & Durand, D. M. Chronic response of the rat sciatic nerve to the flat interface nerve electrode. Ann. Biomed. Eng. 31, 633–642 (2003).
Leventhal, D. K., Cohen, M. & Durand, D. M. Chronic histological effects of the flat interface nerve electrode. J. Neural Eng. 3, 102 (2006).
Tan, D. W. et al. A neural interface provides long-term stable natural touch perception. Sci. Transl. Med. 6, 257ra138 (2014).
Lago, N., Yoshida, K., Koch, K. P. & Navarro, X. Assessment of biocompatibility of chronically implanted polyimide and platinum intrafascicular electrodes. IEEE Trans. Biomed. Eng. 54, 281–290 (2007).
Badia, J. et al. Biocompatibility of chronically implanted transverse intrafascicular multichannel electrode (TIME) in the rat sciatic nerve. IEEE Trans. Biomed. Eng. 58, 2324–2332 (2011).
Branner, A., Stein, R. B., Fernandez, E., Aoyagi, Y. & Normann, R. A. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans. Biomed. Eng. 51, 146–157 (2004).
Christensen, M. B., Wark, H. A. & Hutchinson, D. T. A histological analysis of human median and ulnar nerves following implantation of Utah slanted electrode arrays. Biomaterials 77, 235–242 (2016).
Schady, W., Braune, S., Watson, S., Torebjörk, H. E. & Schmidt, R. Responsiveness of the somatosensory system after nerve injury and amputation in the human hand. Ann. Neurol. 36, 68–75 (1994).
Clippinger, F. W., Avery, R. & Titus, B. R. A sensory feedback system for an upper-limb amputation prosthesis. Bull. Prosthet. Res. 10, 247–258 (1974).
Clippinger, F. W., Seaber, A. V., McElhaney, J. H., Harrelson, J. M. & Maxwell, G. M. Afferent sensory feedback for lower extremity prosthesis. Clin. Orthop. Relat. Res. 169, 202–206 (1982).
Dhillon, G. S., Lawrence, S. M., Hutchinson, D. T. & Horch, K. W. Residual function in peripheral nerve stumps of amputees: implications for neural control of artificial limbs. J. Hand Surg. Am. 29, 605–615 (2004).
Dhillon, G. S. & Horch, K. W. Direct neural sensory feedback and control of a prosthetic arm. IEEE Trans. Neural Syst. Rehabil. Eng. 13, 468–472 (2005).
Valle, G. et al. Comparison of linear frequency and amplitude modulation for intraneural sensory feedback in bidirectional hand prostheses. Sci. Rep. 8, 16666 (2018).
Raspopovic, S. et al. Restoring natural sensory feedback in real-time bidirectional hand prostheses. Sci. Transl. Med. 6, 222ra19 (2014).
Valle, G. et al. Biomimetic intraneural sensory feedback enhances sensation naturalness, tactile sensitivity, and manual dexterity in a bidirectional prosthesis. Neuron https://doi.org/10.1016/j.neuron.2018.08.033 (2018).
George, J. A. et al. Biomimetic sensory feedback through peripheral nerve stimulation improves dexterous use of a bionic hand. Sci. Robot. 4, eaax2352 (2019).
Horch, K., Meek, S., Taylor, T. G. & Hutchinson, D. T. Object discrimination with an artificial hand using electrical stimulation of peripheral tactile and proprioceptive pathways with intrafascicular electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 19, 483–489 (2011).
Zollo, L. et al. Restoring tactile sensations via neural interfaces for real-time force-and-slippage closed-loop control of bionic hands. Sci. Robot. 4, eaau9924 (2019).
Ortiz-Catalan, M., Hakansson, B. & Branemark, R. An osseointegrated human-machine gateway for long-term sensory feedback and motor control of artificial limbs. Sci. Transl. Med. 6, 257re6 (2014).
Oddo, C. M. et al. Intraneural stimulation elicits discrimination of textural features by artificial fingertip in intact and amputee humans. eLife 5, e09148 (2016).
Davis, T. S. et al. Restoring motor control and sensory feedback in people with upper extremity amputations using arrays of 96 microelectrodes implanted in the median and ulnar nerves. J. Neural Eng. 13, 036001 (2016).
Wendelken, S. et al. Restoration of motor control and proprioceptive and cutaneous sensation in humans with prior upper-limb amputation via multiple Utah slanted electrode arrays (USEAs) implanted in residual peripheral arm nerves. J. Neuroeng. Rehabil. 14, 121 (2017).
Rognini, G. et al. Multisensory bionic limb to achieve prosthesis embodiment and reduce distorted phantom limb perceptions. J. Neurol. Neurosurg. Psychiatry 90, 833–836 (2019).
Segil, J. L., Cuberovic, I., Graczyk, E. L., Weir, R. F. ff. & Tyler, D. Combination of simultaneous artificial sensory percepts to identify prosthetic hand postures: a case study. Sci. Rep. 10, 6576 (2020).
Graczyk, E. L., Resnik, L., Schiefer, M. A., Schmitt, M. S. & Tyler, D. J. Home use of a neural-connected sensory prosthesis provides the functional and psychosocial experience of having a hand again. Sci. Rep. 8, 9866 (2018).
Tan, D. W., Schiefer, M. A., Keith, M. W., Anderson, J. R. & Tyler, D. J. Stability and selectivity of a chronic, multi-contact cuff electrode for sensory stimulation in human amputees. J. Neural Eng. 12, 026002 (2015).
Kuiken, T. A. et al. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA 301, 619–628 (2009).
Kuiken, T. A. et al. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: a case study. Lancet 369, 371–380 (2007).
Kuiken, T. A., Marasco, P. D., Lock, B. A., Harden, R. N. & Dewald, J. P. A. Redirection of cutaneous sensation from the hand to the chest skin of human amputees with targeted reinnervation. Proc. Natl Acad. Sci. USA 104, 20061–20066 (2007).
Schofield, J. S., Shell, C. E., Beckler, D. T., Thumser, Z. C. & Marasco, P. D. Long-term home-use of sensory-motor-integrated bidirectional bionic prosthetic arms promotes functional, perceptual, and cognitive changes. Front Neurosci. 14, 120 (2020).
Osborn, L., Betthauser, J., Kaliki, R. & Thakor, N. Live demonstration: targeted transcutaneous electrical nerve stimulation for phantom limb sensory feedback. In 2017 IEEE Biomedical Circuits and Systems Conference (BioCAS) 1-1 (IEEE, 2017); https://doi.org/10.1109/BIOCAS.2017.8325101
Charkhkar, H. et al. High-density peripheral nerve cuffs restore natural sensation to individuals with lower-limb amputations. J. Neural Eng. 15, 056002 (2018).
Petrini, F. M. et al. Sensory feedback restoration in leg amputees improves walking speed, metabolic cost and phantom pain. Nat. Med. 25, 1356–1363 (2019).
Petrini, F. M. et al. Enhancing functional abilities and cognitive integration of the lower limb prosthesis. Sci. Transl. Med. 11, eaav8939 (2019).
Mundell, B. F. et al. The risk of major cardiovascular events for adults with transfemoral amputation. J. Neuroeng. Rehabil. 15, 58 (2018).
Clites, T. R. et al. Proprioception from a neurally controlled lower-extremity prosthesis. Sci. Transl. Med. 10, eaap8373 (2018).
Johansson, R. S. & Flanagan, J. R. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat. Rev. Neurosci. 10, 345–359 (2009).
Marasco, P. D. et al. Illusory movement perception improves motor control for prosthetic hands. Sci. Transl. Med. 10, eaao6990 (2018).
Gandevia, S. C. Illusory movements produced by electrical stimulation of low-threshold muscle afferents from the hand. Brain 108, 965–981 (1985).
Ekedahl, R., Frank, O. & Hallin, R. G. Peripheral afferents with common function cluster in the median nerve and somatotopically innervate the human palm. Brain Res. Bull. 42, 367–376 (1997).
Hagbarth, K. E., Wallen, G. & Löfstedt, L. Muscle spindle activity in man during voluntary fast alternating movements. J. Neurol. Neurosurg. Psychiatry 38, 625–635 (1975).
Stewart, J. D. Peripheral nerve fascicles: anatomy and clinical relevance. Muscle Nerve 28, 525–541 (2003).
D’Anna, E. et al. A closed-loop hand prosthesis with simultaneous intraneural tactile and position feedback. Sci. Robot. 4, eaau8892 (2019).
de la Oliva, N., Mueller, M., Stieglitz, T., Navarro, X. & del Valle, J. On the use of parylene C polymer as substrate for peripheral nerve electrodes. Sci. Rep. 8, 5965 (2018).
Preatoni, G., Valle, G., Petrini, F. M. & Raspopovic, S. Lightening the perceived prosthesis weight with neural embodiment promoted by sensory feedback. Curr. Biol. 31, 1065–1071 (2021).
Gerratt, A. P., Michaud, H. O. & Lacour, S. P. Elastomeric electronic skin for prosthetic tactile sensation. Adv. Funct. Mater. 25, 2287–2295 (2015).
Hua, Q. et al. Skin-inspired highly stretchable and conformable matrix networks for multifunctional sensing. Nat. Commun. 9, 244 (2018).
Lee, W. W. et al. A neuro-inspired artificial peripheral nervous system for scalable electronic skins. Sci. Robot. 4, eaax2198 (2019).
Saal, H. P. & Bensmaia, S. J. Biomimetic approaches to bionic touch through a peripheral nerve interface. Neuropsychologia 79, 344–353 (2015).
Kim, L. H., McLeod, R. S. & Kiss, Z. H. A new psychometric questionnaire for reporting of somatosensory percepts. J. Neural Eng. 15, 013002 (2018).
Hafner, B. J. & Smith, D. G. Differences in function and safety between Medicare functional classification level-2 and-3 transfemoral amputees and influence of prosthetic knee joint control. J. Rehabil. Res. Dev. 46, 417–433 (2009).
Navarro, X. et al. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J. Peripher. Nerv. Syst. 10, 229–258 (2005).
Ochoa, J. & Torebjörk, E. Sensations evoked by intraneural microstimulation of single mechanoreceptor units innervating the human hand. J. Physiol. 342, 633–654 (1983).
Zelechowski, M., Valle, G. & Raspopovic, S. A computational model to design neural interfaces for lower-limb sensory neuroprostheses. J. Neuroeng. Rehabil. 17, 24 (2020).
Raspopovic, S. Advancing limb neural prostheses. Science 370, 290–291 (2020).
Llewellyn, M. E., Thompson, K. R., Deisseroth, K. & Delp, S. L. Orderly recruitment of motor units under optical control in vivo. Nat. Med. 16, 1161–1165 (2010).
Someya, T., Bao, Z. & Malliaras, G. G. The rise of plastic bioelectronics. Nature 540, 379–385 (2016).
Piech, D. K. et al. A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nat. Biomed. Eng. 4, 207–222 (2020).
Abraira, V. E. & Ginty, D. D. The sensory neurons of touch. Neuron 79, 618–639 (2013).
Johansson, R. S. & Flanagan, J. R. in The Senses: A Comprehensive Reference 67–86 (Academic Press, 2008).
Johansson, R. S. & Vallbo, A. B. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J. Physiol. 286, 283–300 (1979).
Johansson, R. S. & Vallbo, Å. B. Tactile sensory coding in the glabrous skin of the human hand. Trends Neurosci. 6, 27–32 (1983).
Yildiz, K. A., Shin, A. Y. & Kaufman, K. R. Interfaces with the peripheral nervous system for the control of a neuroprosthetic limb: a review. J. Neuroeng. Rehabil. 17, 43 (2020).
Benvenuto, A. et al. Intrafascicular thin-film multichannel electrodes for sensory feedback: evidences on a human amputee. In 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology 1800–1803 (IEEE, 2010).
Negi, S., Bhandari, R., Rieth, L. & Solzbacher, F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed. Mater. 5, 15007 (2010).
Badia, J. et al. in Direct Nerve Stimulation for Induction of Sensation and Treatment of Phantom Limb Pain 155–169 (River Publishers, 2019).
Graczyk, E. L., Delhaye, B. P., Schiefer, M. A., Bensmaia, S. J. & Tyler, D. J. Sensory adaptation to electrical stimulation of the somatosensory nerves. J. Neural Eng. 15, 046002 (2018).
Strauss, I. et al. Characterization of multi-channel intraneural stimulation in transradial amputees. Sci. Rep. 9, 9258 (2019).
Kluger, D. T. et al. Virtual reality provides an effective platform for functional evaluations of closed-loop neuromyoelectric control. IEEE Trans. Neural Syst. Rehabil. Eng. 27, 876–886 (2019).
Acknowledgements
We acknowledge support from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme FeelAgain (grant agreement no. 759998), by H2020-EIC-FTI-2018-2020 GoSafe (grant agreement no. 870144), by the Swiss National Science Foundation (SNSF) and Innosuisse under the Bridge Proof of Concept programme (MYLEG no. 193724), SNSF grant MOVEIT (no. 205321_197271) and Innosuisse grant (no. 47462.1 IP-ICT). The funders had no role in the manuscript preparation or submission.
Author information
Authors and Affiliations
Contributions
S.R., G.V. and F.M.P. conceived the Review, edited the manuscript and made the figures. S.R. wrote the section ‘The role of materials in the development of neuroprostheses’. G.V. wrote the section ‘Bionic limb applications’. F.M.P. wrote the section ‘Sensory feedback is an unmet need for prosthetic users’. S.R., G.V. and F.M.P. wrote the section ‘Prospective and big picture’. All authors authorized submission of the manuscript, but the final submission decision was made by the corresponding author.
Corresponding author
Ethics declarations
Competing interests
S.R. and F.M.P. hold shares of SensArs Neuroprosthetics Sarl, a start-up company dealing with the commercialization of neurocontrolled artificial limbs. G.V. declares no competing interests.
Additional information
Peer review information Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raspopovic, S., Valle, G. & Petrini, F.M. Sensory feedback for limb prostheses in amputees. Nat. Mater. 20, 925–939 (2021). https://doi.org/10.1038/s41563-021-00966-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-021-00966-9
This article is cited by
-
Biomimetic computer-to-brain communication enhancing naturalistic touch sensations via peripheral nerve stimulation
Nature Communications (2024)
-
Shaping high-performance wearable robots for human motor and sensory reconstruction and enhancement
Nature Communications (2024)
-
Neuromorphic hardware for somatosensory neuroprostheses
Nature Communications (2024)
-
Thermally sentient bionic limbs
Nature Biomedical Engineering (2024)
-
Integration of proprioception in upper limb prostheses through non-invasive strategies: a review
Journal of NeuroEngineering and Rehabilitation (2023)