Building a better fuel cell begins with surface chemistry

Fuel cells powered by electrocatalytic reactions have the potential to eliminate pollution caused by burning fossil fuels, if they could be made more efficient. Key to higher efficiency are the chemical reactions at the surfaces of the materials involved. An international team of scientists peered deep into the molecular reactions of ethanol on gold surfaces in alkaline environments typically seen in model fuel cells.
Fuel cells convert chemical energy into clean electrical energy through a series of reactions. Changes in surface chemistry during these reactions may influence both the catalytic efficiency and the reactions themselves. By providing fundamental insight into surface chemistry, this work is giving scientists a more complete picture of the catalytic process and will help them design better fuel cells that can be used to power a single device such as your laptop computer or a local electric grid.
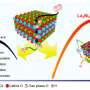
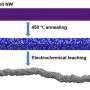
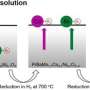
Scientists from the Chinese Academy of Sciences, China's National Centre for Mass Spectrometry, and EMSL, the Environmental Molecular Sciences Laboratory, a U.S. Department of Energy Office of Science user facility, designed and fabricated a high-powered way to visualize the transformation of thin gold surfaces inside a direct alcohol fuel cell. This work made use of EMSL's time-of-flight secondary ion mass spectrometer, and allowed the team to peer into the molecular functioning of the catalytic reactions. This work provided direct molecular evidence of the changes gold undergoes in these reactions. The scientists also identified additional active sites—places on the surface where the needed conversion can take place. These and other insights will provide useful information to optimize fuel cell efficiency.
More information: Yanyan Zhang et al. Potential-Dynamic Surface Chemistry Controls the Electrocatalytic Processes of Ethanol Oxidation on Gold Surfaces, ACS Energy Letters (2018). DOI: 10.1021/acsenergylett.8b02019
Provided by Environmental Molecular Sciences Laboratory