A paper focusing on the role of co-chaperones involved in ribonucleotide reductase (RNR) activity was recently published in PLOS Genetics and made reference to StressMarq’s Mouse Anti-Yeast HSP40, YDJ1 Antibody.
RNR is an enzyme that converts ribonucleotides (NTPs) to deoxyribonucleotides (dNTPs). This conversion is essential for DNA synthesis making RNR an attractive target for anti-cancer therapies, as inhibiting RNR can promote apoptosis.
Molecular chaperones are proteins that support the folding/unfolding or assembly/disassembly of other proteins, known as “clients.” One such chaperone is the heat shock protein 70 (Hsp70). Unstable oncoproteins use this extensively and, as such, it is highly expressed in particular types of cancer.
HSP70 Crystal Structure. Image Credit: StressMarq Biosciences
A chaperone of RNR, Hsp70 also has other co-chaperones. The purpose of this paper is to investigate the co-chaperones that are involved with the RNR complex. Damaged DNA is repaired by the dNTPs that this produces. RNR could be prevented from repairing DNA and the cell cycle prevented from progressing if these co-chaperones are inhibited. This is an ideal scenario for cancer cells.
RNR is inhibited by the small molecule hydroxyurea (HU) as it reduces a tyrosyl radical in R2, an RNR subunit. Cancer and sickle-cell disease can be treated with this, and it is able to stall the DNA replication fork to generate single-stranded DNA.
Hydroxyurea Chemical Structure. Image Credit: CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=657995
The RNR complex is destabilized and HU and other RNR inhibitors can have a greater effect when Hsp70 or Hsp90 are inhibited. Researchers at UNC Charlotte screened 28 yeast co-chaperone knockout strains for sensitivity to HU in order to identify other targets that may increase the effect of RNR inhibitors, such as HU.
If an increased sensitivity to HU was displayed by yeast without a certain co-chaperone then that co-chaperone was probably involved in RNR’s response to DNA damage. If yeast cells lacked Ydj1, a HSP40, they were destroyed at lower concentrations of HU meaning that they were more sensitive to HU than wild-type and other mutant yeast strains.
The role of Ydj1 was tested by researchers in the DNA damage response pathway by carrying out a β-galactosidase activity assay and making a comparison with the responses of wild-type and ydj1Δ cells to RNR3 promoter-lacZ, a DNA damage response-promoter. If there is DNA damage then the gene RNR3 will be expressed in response.
As anticipated, cells lacking ydj1 had impaired RNR3 transcription. This suggested that the DNA damage response was compromised. As such, Ydj1 is necessary for the DNA damage response and associated resistance to HU-induced cell death.
ydj1Δ cells were also shown to decrease RNR2 and RNR4 transcription (and in turn decrease levels of RNR subunits Rnr2 and Rnr4). In ydj1Δ cells treated with HU Rnr2 was significantly destabilized. Ydj1 was found to interact with Rnr2 in yeast. HDJ2, its human equivalent, was found to interact with R2B, a subunit of RNR.
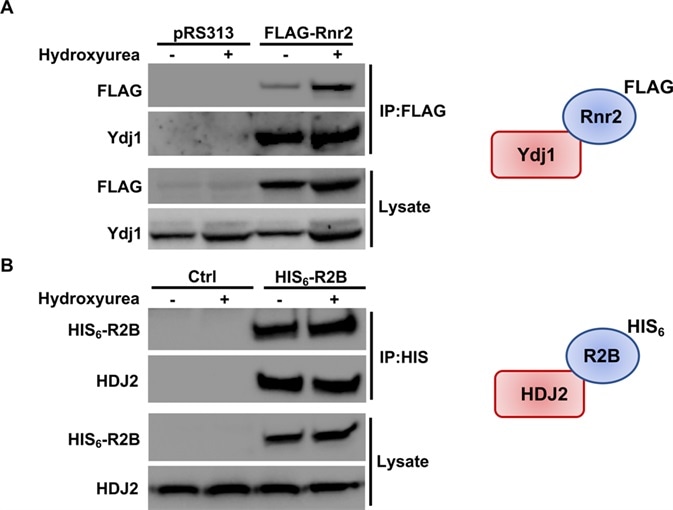
(A) Rnr2 interacts with Ydj1 in yeast. WT cells transformed with either pRS313 or plasmid expressing FLAG-tagged Rnr2 were grown to exponential phase and were either left untreated or were treated with HU as in Fig 3. Cell extracts (lysate) and immunoprecipitates (IP) with anti-FLAG M2 magnetic beads were subjected to SDS-PAGE and analyzed by immunoblotting with anti-FLAG antibodies to detect Rnr2 or anti-Ydj1 antibodies to detect Ydj1. (B) R2B interacts with HDJ2 in mammalian cells. HEK293 cells were transfected with a plasmid expressing CMV-driven HIS6-tagged R2B. Cells extracts were obtained 48 hours post-transfection. Cell extracts (lysate) and immunoprecipitates (IP) with HIS-dynabeads were subjected to SDS-PAGE and analyzed by immunoblotting with tetra-HIS antibodies to detect R2B or anti-HDJ2 antibodies to detect HDJ2. Image Credit: PLOS Genetics https://journals.plos.org/plosgenetics/article/metrics?id=10.1371/journal.pgen.1007462
116-9e is a small molecule that blocks Hsp40-Hsp70 binding. When HEK293 cells were treated with this it disrupted the R2B-Hsp70 interaction. Hsp40s transport clients to Hsp70s for folding. As such, if the interaction between HDJ2 and Hsp70 is disrupted, this may prevent cells from resisting the effects of HU through RNR-enabled DNA repair. A synergistic effect was displayed by 116-9e and HU, which inhibited growth in HAP1 cells, suggesting that a two-pronged approach of HDJ2 and RNR inhibitors may provide a promising cancer treatment option.
Hsp70 is a well-conserved molecular chaperone involved in the folding, stabilization, and eventual degradation of many “client” proteins. Hsp70 is regulated by a suite of co-chaperone molecules that assist in Hsp70-client interaction and stimulate the intrinsic ATPase activity of Hsp70.
While previous studies have shown the anticancer target ribonucleotide reductase (RNR) is a client of Hsp70, the regulatory cochaperones involved remain to be determined. To identify co-chaperone(s) involved in RNR activity, 28 yeast co-chaperone knockout mutants were screened for sensitivity to the RNR-perturbing agent Hydroxyurea. Ydj1, an important cytoplasmic Hsp70 co-chaperone was identified to be required for growth on HU. Ydj1 bound the RNR subunit Rnr2 and cells lacking Ydj1 showed a destabilized RNR complex.
Suggesting broad conservation from yeast to human, HDJ2 binds R2B and regulates RNR stability in human cells. Perturbation of the Ssa1-Ydj1 interaction through mutation or Hsp70-HDJ2 via the small molecule 116-9e compromised RNR function, suggesting chaperone dependence of this novel role. Mammalian cells lacking HDJ2 were significantly more sensitive to RNR inhibiting drugs such as hydroxyurea, gemcitabine and triapine. Taken together, this work suggests a novel anticancer strategy-inhibition of RNR by targeting Hsp70 co-chaperone function.
We are really thankful to StressMarq for generating antibodies for Ydj1. We used this antibody for Immunoprecipitation assay followed by western blot to demonstrate that Ydj1 interacts with Rnr2.
This work was recently published in PLOS Genetics and StressMarq antibody really helped us in our experiments. Apart from this, we work on other chaperones such as Hsp110 and I have used the polyclonal Hsp110 antibody. These are both excellent antibodies.
Nitika, a PhD Student, The Truman Lab, UNC Charlotte
Acknowledgments
Produced from materials originally authored by Patricia Thomson from StressMarq Biosciences Inc.
About StressMarq Biosciences
Established in 2007, StressMarq Biosciences Inc. is a supplier of life science products that operates out of Victoria, Canada with a small, but dedicated group of scientists. Headed by our CEO and President Dr. Ariel Louwrier, StressMarq provides the research community with high-quality reagents backed with rigorous quality control data, expert scientific support, and fast international delivery.
“Discovery through partnership, Excellence through quality”
With over 7,000 products, our growth can be attributed to the continual production of cutting edge research products. Our diverse portfolio of primary antibodies, antibody conjugates, proteins, immunoassay kits and small molecules bridges across the life sciences, including products for cancer research, cardiovascular disease, cell signaling and neuroscience. To aid research worldwide, StressMarq has an extensive network of international distributors that allow us to supply reagents to over 50 countries.
In the years to come, StressMarq will continue to aid life science research by providing “Discovery through partnership, and Excellence through quality”.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.