Abstract
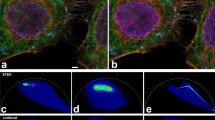
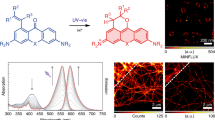
Illumination of fluorophores can induce a loss of the ability to fluoresce, known as photobleaching. Interestingly, some fluorophores photoconvert to a blue-shifted fluorescent molecule as an intermediate on the photobleaching pathway, which can complicate multicolor fluorescence imaging, especially under the intense laser irradiation used in super-resolution fluorescence imaging. Here, we discuss the mechanisms of photoblueing of fluorophores and its impact on fluorescence imaging, and show how it can be prevented.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data that support the findings described in this study are available in the manuscript and the related supplementary information, and from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Foote, C. S. Mechanisms of photosensitized oxidation. Science 162, 963–970 (1968).
Grewer, C. & Brauer, H.-D. Mechanisms of the triplet-state quenching by molecular oxygen in solution. J. Phys. Chem. 98, 4230–4235 (1994).
Giloh, H. & Sedat, J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science 217, 1252–1255 (1982).
Schermelleh, L. et al. Super-resolution microscopy demystified. Nat. Cell Biol. 21, 72–84 (2019).
Evans, N. A. Photofading of rhodamine dyes: I – identification of some rhodamine B photoproducts. J. Soc. Dyers Colour. 86, 174–177 (1970).
Li, X., Liu, G. & Zhao, J. Two competitive primary processes in the photodegradation of cationic triarylmethane dyes under visible irradiation in TiO2 dispersions. New J. Chem. 23, 1193–1196 (1999).
Butkevich, A. N., Bossi, M. L., Lukinavičius, G. & Hell, S. W. Triarylmethane fluorophores resistant to oxidative photobluing. J. Am. Chem. Soc. 141, 981–989 (2019).
Freundt, E. C., Czapiga, M. & Lenardo, M. J. Photoconversion of Lysotracker Red to a green fluorescent molecule. Cell Res. 17, 956–958 (2007).
van de Linde, S. et al. Direct stochastic optical reconstruction microscopy with standard fluorescent probes. Nat. Protoc. 6, 991–1009 (2011).
Dempsey, G. T., Vaughan, J. C., Chen, K. H., Bates, M. & Zhuang, X. Evaluation of fluorophores for optimal performance in localization-based super-resolution imaging. Nat. Methods 8, 1027–1036 (2011).
Byers, G. W., Gross, S. & Henrichs, P. M. Direct and sensitized photooxidation of cyanine dyes. Photochem. Photobiol. 23, 37–43 (1976).
Nani, R. R., Kelley, J. A., Ivanic, J. & Schnermann, M. J. Reactive species involved in the regioselective photooxidation of heptamethine cyanines. Chem. Sci. 6, 6556–6563 (2015).
Stone, M. B. & Veatch, S. L. Far-red organic fluorophores contain a fluorescent impurity. ChemPhysChem 15, 2240–2246 (2014).
Dirix, L. et al. Photoconversion of far-red organic dyes: implications for multicolor super-resolution imaging. ChemPhotoChem 2, 433–441 (2018).
Kwok, S. J. J. et al. Two-photon excited photoconversion of cyanine-based dyes. Sci. Rep. 6, 23866 (2016).
Liao, Z. et al. Green emitting photoproducts from terrylene diimide after red illumination. J. Am. Chem. Soc. 135, 19180–19185 (2013).
Sanborn, M. E., Connolly, B. K., Gurunathan, K. & Levitus, M. Fluorescence photophysics and properties of the sulfoindocyanine Cy3 linked covalently to DNA. J. Phys. Chem. B 111, 11064–11074 (2007).
Yanagida, T., Nakase, M., Nishiyama, K. & Oosawa, F. Direct observation of motion of single F-actin filaments in the presence of myosin. Nature 307, 58–60 (1984).
Funatsu, T., Harada, Y., Tokunaga, M., Salto, K. & Yanagida, T. Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature 374, 555–559 (1995).
Rasnik, I., McKinney, S. A. & Ha, T. Nonblinking and long-lasting single-molecule fluorescence imaging. Nat. Methods 3, 891–893 (2006).
Wäldchen, S., Lehmann, J., Klein, T., van de Linde, S. & Sauer, M. Light-induced cell damage in live-cell super-resolution microscopy. Sci. Rep. 5, 15348 (2015).
DeRosa, M. C. & Crutchley, R. J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 233–234, 351–371 (2002).
Ando, R., Mizuno, H. & Miyawaki, A. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science 306, 1370–1373 (2004).
Winkel, B. M. F. et al. A tracer-based method enables tracking of Plasmodium falciparum malaria parasites during human skin infection. Theranostics 9, 2768–2778 (2019).
Acknowledgements
S.S.M. and M.J.S. were supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute (NCI), Center for Cancer Research. We thank the Biophysics Resource in the Structural Biophysics Laboratory, Center for Cancer Research, NCI at Frederick for assistance with liquid chromatography–mass spectrometry analysis. D.A.H., G.B. and M.S. acknowledge financial support from the Deutsche Forschungsgemeinschaft (DFG SA829/19-1 and TRR 166 ReceptorLight, project A04) and the European Regional Development Fund (EFRE project, Center for Personalized Molecular Immunotherapy).
Author information
Authors and Affiliations
Contributions
M.S., G.B. and M.J.S. conceived the experiments. D.A.H., G.B. and S.S.M. prepared samples, performed measurements and analyzed data. M.S. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Methods thanks Susana Rocha and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Rita Strack was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
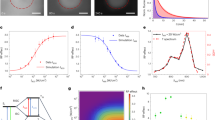
Extended Data Fig. 1 Fluorescence decays and exponential fits of Cy5 before and after irradiation.
The photoconverted (photobleached) product was purified by HPLC. The fluorescence decays were recorded at the emission maxima of 670 and 565 nm, respectively, in PBS, pH 7.4. The lower panels show residual differences between experimental and fitted values. We measured main lifetime components of 0.93 ns with an amplitude > 98% before irradiation and 0.32 ns with an amplitude > 98% for the photoconverted product.
Supplementary information
Supplementary Information
Supplementary Note 1.
Source data
Source Data Fig. 1
Spectroscopic raw data of all figures
Source Data Extended Data Fig. 1
Source data for Extended Data Fig. 1
Rights and permissions
About this article
Cite this article
Helmerich, D.A., Beliu, G., Matikonda, S.S. et al. Photoblueing of organic dyes can cause artifacts in super-resolution microscopy. Nat Methods 18, 253–257 (2021). https://doi.org/10.1038/s41592-021-01061-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-021-01061-2
This article is cited by
-
Fluorescence resonance energy transfer at the single-molecule level
Nature Reviews Methods Primers (2024)
-
Quantitatively mapping local quality of super-resolution microscopy by rolling Fourier ring correlation
Light: Science & Applications (2023)
-
Single molecule imaging simulations with advanced fluorophore photophysics
Communications Biology (2023)
-
Single-molecule localization microscopy reveals the ultrastructural constitution of distal appendages in expanded mammalian centrioles
Nature Communications (2023)