Abstract
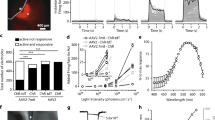
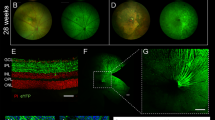
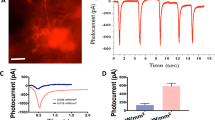
Uveal melanoma is the most common intraocular primary malignancy in adults and has been considered a fatal disease for decades. Optogenetics is an emerging technique that can control the activation of signaling components via irradiation with visible light. The clinical translation of optogenetics has been limited because of the need for surgical implantation of electrodes and relatively shallow tissue penetration. As visible light easily penetrates the eyes, we hypothesized that an optogenetics approach can be an effective treatment of uveal melanoma without surgery. In this study, we evaluated the feasibility of this strategy by using a genetically encoded optogenetic system based on reversible blue light-induced binding pairs between Fas-CIB1-EGFP and CRY2-mCherry-FADD. Subretinal injection of B16 cells was performed to create a uveal melanoma model. Plasmids pairs were co-transfected into B16 cells. We found that blue light irradiation dynamically controlled the translocation of FADD to Fas on the plasma membrane and induced the apoptosis of B16 cells transfected with the optogenetic nanosystem in vitro. Moreover, the blue light-controlled optogenetic nanosystem suppressed the growth of uveal melanoma in vivo by inducing apoptosis. These results suggest that light-controlled optogenetic therapy can be used as a potential novel therapeutic strategy for uveal melanoma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Amaro A, Gangemi R, Piaggio F, Angelini G, Barisione G, Ferrini S, et al. The biology of uveal melanoma. Cancer Metast Rev. 2017;36:109–40.
Shain AH, Bagger MM, Yu R, Chang D, Liu S, Vemula S, et al. The genetic evolution of metastatic uveal melanoma. Nat Genet. 2019;51:1123–30.
Sagoo MS, Harbour JW, Stebbing J, Bowcock AM. Combined PKC and MEK inhibition for treating metastatic uveal melanoma. Oncogene. 2014;33:4722–3.
Damato BE. Treatment selection for uveal melanoma. Dev Ophthalmol. 2012;49:16–26.
Liu YM, Li Y, Wei WB, Xu X, Jonas JB. Clinical characteristics of 582 patients with uveal melanoma in China. Plos ONE. 2015;10:e144562.
Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, et al. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell. 2014;25:822–30.
Moore AR, Ceraudo E, Sher JJ, Guan Y, Shoushtari AN, Chang MT, et al. Recurrent activating mutations of G-protein-coupled receptor CYSLTR2 in uveal melanoma. Nat Genet. 2016;48:675–80.
Venza I, Visalli M, Beninati C, Benfatto S, Teti D, Venza M. IL-10Ralpha expression is post-transcriptionally regulated by miR-15a, miR-185, and miR-211 in melanoma. BMC Med Genomics. 2015;8:81.
Chen X, He D, Dong XD, Dong F, Wang J, Wang L, et al. MicroRNA-124a is epigenetically regulated and acts as a tumor suppressor by controlling multiple targets in uveal melanoma. Invest Ophthalmol Vis Sci. 2013;54:2248–56.
Oliva M, Rullan AJ, Piulats JM. Uveal melanoma as a target for immune-therapy. Ann Transl Med. 2016;4:172.
Luo C, Shen J. Research progress in advanced melanoma. Cancer Lett. 2017;397:120–6.
Alvarez-Rodriguez B, Latorre A, Posch C, Somoza A. Recent advances in uveal melanoma treatment. Med Res Rev. 2017;37:1350–72.
Ye H, Fussenegger M. Optogenetic medicine: synthetic therapeutic solutions precision-guided by light. Csh Perspect Med. 2019;9:a034371.
Deisseroth K. Optogenetics. Nat Methods. 2011;8:26–9.
Kim CK, Adhikari A, Deisseroth K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci. 2017;18:222–35.
Jiang C, Li HT, Zhou YM, Wang X, Wang L, Liu ZQ. Cardiac optogenetics: a novel approach to cardiovascular disease therapy. Europace. 2018;20:1741–9.
Shao J, Xue S, Yu G, Yu Y, Yang X, Bai Y, et al. Smartphone-controlled optogenetically engineered cells enable semiautomatic glucose homeostasis in diabetic mice. Sci Transl Med. 2017;9:eaal2298.
Kennedy MJ, Hughes RM, Peteya LA, Schwartz JW, Ehlers MD, Tucker CL. Rapid blue-light-mediated induction of protein interactions in living cells. Nat Methods. 2010;7:973–5.
Konermann S, Brigham MD, Trevino A, Hsu PD, Heidenreich M, Cong L, et al. Optical control of mammalian endogenous transcription and epigenetic states. Nature. 2013;500:472–6.
Zhang K, Cui B. Optogenetic control of intracellular signaling pathways. Trends Biotechnol. 2015;33:92–100.
Zhang K, Duan L, Ong Q, Lin Z, Varman PM, Sung K, et al. Light-mediated kinetic control reveals the temporal effect of the Raf/MEK/ERK pathway in PC12 cell neurite outgrowth. Plos ONE. 2014;9:e92917.
Yu L, Zhou X, Zhou L, Cao G, Po SS, Huang B, et al. Optogenetic modulation of cardiac sympathetic nerve activity to prevent ventricular arrhythmias. J Am Coll Cardiol. 2017;70:2778–90.
Chen S, Weitemier AZ, Zeng X, He L, Wang X, Tao Y, et al. Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science. 2018;359:679–84.
Zheng B, Wang H, Pan H, Liang C, Ji W, Zhao L, et al. Near-Infrared light triggered upconversion optogenetic nanosystem for cancer therapy. ACS Nano. 2017;11:11898–907.
Tanaka Y. Impact of near-infrared radiation in dermatology. World J Dermatol. 2012;1:30–7.
Konig K, Liang H, Berns MW, Tromberg BJ. Cell damage in near-infrared multimode optical traps as a result of multiphoton absorption. Opt Lett. 1996;21:1090–2.
Chen L, Sun B, Zhang S, Zhao X, He Y, Zhao S, et al. Influence of Microenvironments On Microcirculation Patterns and Tumor Invasion-Related Protein Expression in Melanoma. Oncol Rep. 2009;21:917–23.
He L, Zhang Y, Ma G, Tan P, Li Z, Zang S, et al. Near-infrared photoactivatable control of Ca(2+) signaling and optogenetic immunomodulation. Elife. 2015;4:e10024.
Xu Y, Hyun YM, Lim K, Lee H, Cummings RJ, Gerber SA, et al. Optogenetic control of chemokine receptor signal and T-cell migration. Proc Natl Acad Sci USA. 2014;111:6371–6.
Zhang Y, Ivanova E, Bi A, Pan ZH. Ectopic expression of multiple microbial rhodopsins restores ON and OFF light responses in retinas with photoreceptor degeneration. J Neurosci. 2009;29:9186–96.
Canal-Fontcuberta I, Salomao DR, Robertson D, Cantrill HL, Koozekanani D, Rath PP, et al. Clinical and histopathologic findings after photodynamic therapy of choroidal melanoma. Retina. 2012;32:942–8.
Jmor F, Hussain RN, Damato BE, Heimann H. Photodynamic therapy as initial treatment for small choroidal melanomas. Photodiagnosis Photodyn Ther. 2017;20:175–81.
Blasi MA, Pagliara MM, Lanza A, Sammarco MG, Caputo CG, Grimaldi G, et al. Photodynamic therapy in ocular oncology. Biomedicines. 2018;6:17.
Zheng B, Bai Y, Chen H, Pan H, Ji W, Gong X, et al. Targeted delivery of tungsten oxide nanoparticles for multifunctional anti-tumor therapy via macrophages. Biomater Sci. 2018;6:1379–89.
Acknowledgements
We thank Prof. Colin J. Barnstable for his contribution in revising this manuscript. This work was supported by grants from the National Natural Science Foundation of China (81900894, 81671642, 81870651) and Natural Science Foundation of Tianjin (18JCQNJC11300).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, M., Lin, X., Zhang, J. et al. Blue light-triggered optogenetic system for treating uveal melanoma. Oncogene 39, 2118–2124 (2020). https://doi.org/10.1038/s41388-019-1119-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-1119-5
This article is cited by
-
Optogenetic control of NOTCH1 signaling
Cell Communication and Signaling (2022)
-
Cell Rover—a miniaturized magnetostrictive antenna for wireless operation inside living cells
Nature Communications (2022)